Immunity is a physiological protective mechanism of the body, fundamentally aimed at distinguishing self from non-self. Its primary functions include the defense against infections, the removal of aging, damaged, or dying cells, and the recognition and clearance of mutated cells to maintain internal homeostasis. Dysfunctions in immune regulation can result in abnormal immune responses, leading not only to immunodeficiency—characterized by increased susceptibility to infections and impaired immune surveillance associated with malignancies—but also to allergic reactions, autoimmune conditions, and excessive inflammatory responses.
Characteristics of Immune System Development in Children
The immune system of children differs significantly from that of adults, which contributes to the unique features of immunological diseases in children. Traditionally, it has been believed that the immune system, particularly in neonates, is immature. However, at birth, the immune organs and cells are relatively well-developed. Reduced immune function primarily results from the lack of antigen exposure and the absence of established immune memory.
Monocytes/Macrophages
Neonatal monocytes are fully developed, yet their function is suboptimal due to a lack of accessory factors. Consequently, chemotaxis, adhesion, phagocytosis, oxidative bactericidal activity, and the production of G-CSF, IL-8, IL-6, IFN-γ, IL-12, and antigen presentation capabilities are weaker compared to adults. The type and dosage of antigens or allergens encountered during the neonatal period directly influence the immune regulatory functions of monocytes/macrophages, especially dendritic cells (DCs), and can significantly affect immune status later in life.
Neutrophils
Neutrophil counts in peripheral blood are elevated within the first 12 hours after birth due to the stress of labor but decline over the next 72 hours before gradually increasing to reach adult levels. The limited neutrophil reservoir in neonates predisposes them to neutropenia during severe neonatal sepsis. Immature neonates and those born via cesarean section exhibit reduced expression of chemotactic and adhesion molecules, such as Mac-1 (CD11b/CD18), CD10, CD13, and CD33. Additionally, FcRIII expression in neutrophils is reduced in preterm infants and does not reach adult levels until 2 weeks after birth. Temporary functional impairments in neutrophils render neonates more susceptible to pyogenic infections.
T Lymphocytes and Cytokines
T Cell Quantity
Mature T cells constitute up to 80% of peripheral lymphocytes. Peripheral lymphocyte counts can, therefore, reflect T cell numbers. At birth, lymphocyte counts are relatively low, but they surpass neutrophil percentages by days 4–6. By 4–6 years of age, the proportions of lymphocytes and neutrophils become comparable, and lymphocyte numbers gradually decline with age to reach low levels in later life.
T Cell Phenotype and Function
The majority of T cells (97%) in cord blood are CD45RA+ "naive" T cells (compared to 50% in adult peripheral blood), with very few CD45RO+ memory T cells. Neonatal T cells exhibit weaker expression of CD25 and CD40 ligands than adult T cells, with reduced capacity to assist B cells in synthesizing and class-switching immunoglobulins (Ig), promoting phagocytes, and activating cytotoxic T lymphocytes (CTLs).
Th Subpopulations
During pregnancy, to prevent maternal-fetal immune rejection, maternal Th2 cells predominate over Th1 cells. This Th2-dominant state persists for a short time in neonates.
Cytokines
Cytokine production by neonatal T cells—including TNF and GM-CSF—is only 50% of adult levels. IFN-γ, IL-10, and IL-4 levels are 10%–20% of adult levels but increase gradually upon repeated antigen stimulation. IFN-γ reaches adult levels by approximately 175 days of life.
NK Cells and Antibody-Dependent Cellular Cytotoxicity (ADCC)
NK cell surface marker CD56 is nearly absent at birth and remains low throughout the neonatal period. NK cell activity reaches adult levels between 1 and 5 months of age. ADCC is only 50% of adult function at birth and matures to adult levels by 1 year of age.
B Lymphocytes and Immunoglobulins (Igs)
B Cell Phenotype and Function
Without prior antigen exposure, fetal and neonatal B cells produce IgM but not IgG or IgA. B cells capable of secreting IgG reach adult levels at age 2, while those producing IgA reach adult levels by age 5. Due to insufficient Th cell function, neonatal B cells fail to generate antibodies against capsulated polysaccharide bacteria.
IgG
IgG is the only immunoglobulin that crosses the placenta, using an active transport mechanism. A large amount of IgG transfer occurs late in gestation. Preterm infants (<32 weeks of gestation) have serum IgG levels <400 mg/dl, while full-term infants have IgG serum concentrations 5%–10% higher than their mothers. Neonatal IgG synthesis is slower than IgM, with serum IgG levels reaching their lowest point at 3–5 months of age. By 10–12 months, all IgG in the serum is self-produced, and adult levels are reached by 8–10 years of age. IgG subclasses, such as IgG2 (specific for bacterial polysaccharides), increase with age but slowly during the first 2 years, increasing susceptibility to capsulated bacterial infections.
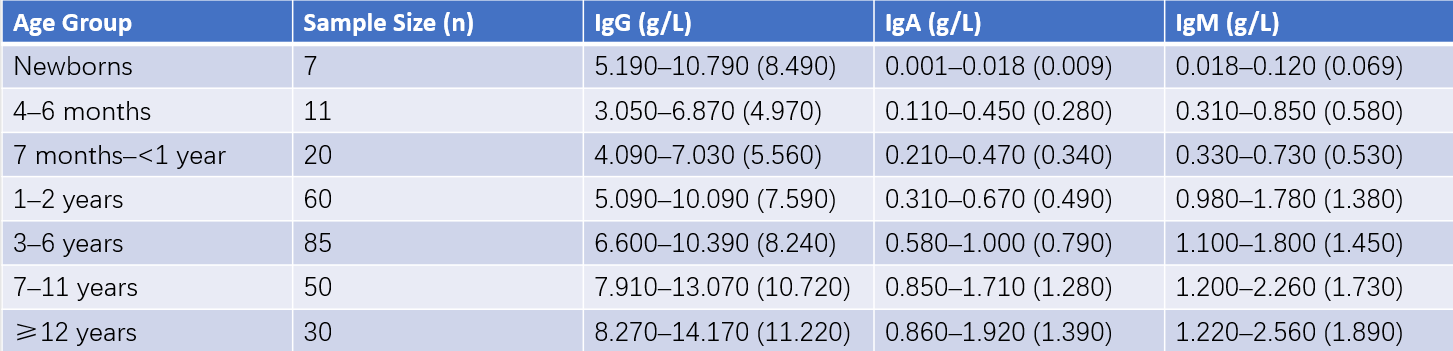
Table 1 Serum immunoglobulin levels in healthy children
Note: The numbers in parentheses represent the mean value, while the ranges denote the mean ±2SD.
IgM
The fetus begins producing IgM during gestation, with rapid increases after birth. Boys reach adult serum levels by age 3, while girls reach these levels by age 6. Elevated cord IgM levels suggest intrauterine infection.
IgA
IgA maturation is the slowest of all immunoglobulins, with adult levels reached during adolescence or young adulthood. Secretory SIgA is undetectable in neonates but measurable in saliva by 2 months and reaches adult levels by 2–4 years of age.
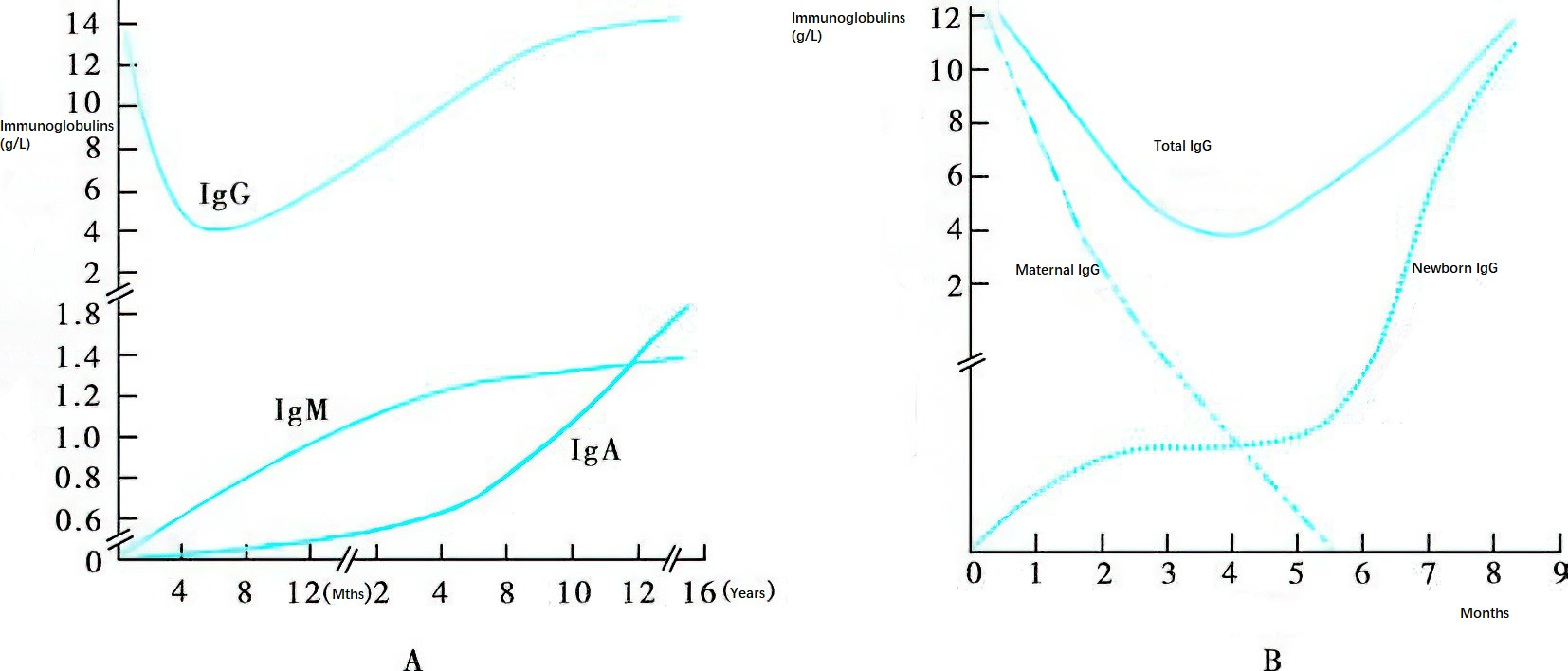
Figure 1 Ontogeny of immunoglobulins
A. Ontogeny of IgG, IgM, and IgA
Due to the placental transfer of maternal IgG, newborns exhibit high serum IgG levels at birth. Maternal IgG gradually disappears, and the serum IgG concentration decreases to its lowest point around 3–5 months of age. Production of endogenous IgG in infants gradually increases, reaching adult levels by approximately 8–10 years of age. IgM and IgA levels are nearly undetectable at birth. Among these, IgM shows the most rapid development, reaching adult levels at 6–8 years of age. IgA levels approach adult concentrations by 11–12 years of age.
B. Dynamic Changes in Serum IgG in Infants Within the First 9 Months Post-Birth
The graph depicts the serum IgG levels sourced from maternal IgG and newly synthesized IgG by the newborn over the first 9 months after birth.
Complement System and Other Immune Components
Complement
Maternal complement components are not transferred to the fetus. Neonatal complement activity, including CH50, C3, C4, and C5 from the classical pathway, is 50%–60% of maternal levels and reaches adult levels by 3–6 months of age. Components of the alternative pathway are even more underdeveloped, with B factor and properdin representing only 35%–60% and 35%–70% of adult levels, respectively. Preterm infants have lower levels of both classical and alternative pathway components compared to term infants.
Other Immune Molecules
Neonatal plasma fibronectin concentrations are only 1/3–1/2 of adult levels and are lower in preterm infants. Similarly, mannose-binding lectin (MBL) levels are reduced in preterm neonates and reach the levels of full-term neonates by 10–20 weeks of age.
To be continued