Congenital megacolon, also known as Hirschsprung disease (HD) or aganglionic megacolon, is a common developmental anomaly of the digestive tract. Its incidence is second only to that of congenital anorectal malformations and has a tendency for familial occurrence. The prevalence is approximately 1 in 5,000 live births, with a higher frequency in males. The male-to-female ratio is about 4:1. Clinically, it is characterized by persistent constipation and the absence of ganglion cells in the affected intestinal segment.
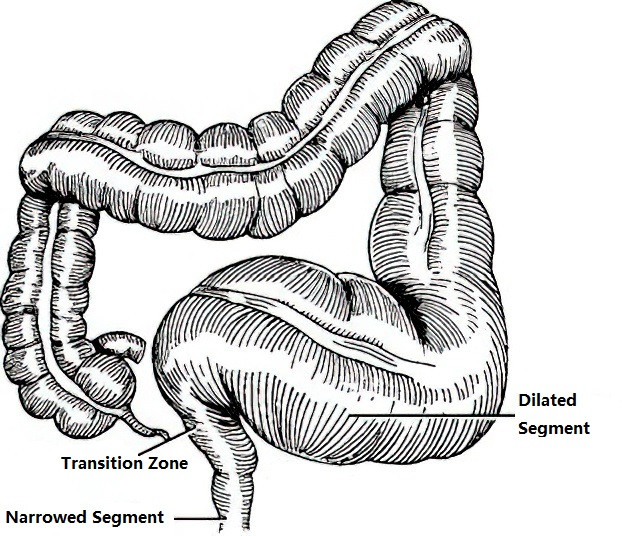
Figure 1 Congenital megacolon
The condition arises due to a failure in the migration and development of neuroectoderm-derived neural crest cells, resulting in the absence of ganglion cells in the myenteric plexus of the distal colon. This leads to continuous spastic narrowing of the affected bowel segment, which in turn causes dilation and thickening of the proximal bowel, functional intestinal obstruction, and subsequent complications. The primary pathology lies in the narrowed distal segment, rather than the dilated and hypertrophied proximal segment. The length of the aganglionic segment may vary, resulting in the classification of congenital megacolon into long-segment and short-segment types.
Clinical Manifestations
Most neonates with congenital megacolon experience acute intestinal obstruction within the first week after birth. In approximately 90% of cases, meconium is not passed within 24–48 hours or only a small amount is discharged, requiring interventions such as enemas to allow passage of larger amounts of meconium. In addition to delayed or absent meconium passage, symptoms typically include persistent constipation, abdominal distension, and vomiting.
Digital rectal examination provides valuable diagnostic clues. Findings include an empty rectal ampulla, with feces retained in the dilated colon above. The examination may trigger a defecation reflex, leading to the sudden expulsion of a large amount of stool and gas, which relieves abdominal distension. During infancy, constipation worsens progressively and becomes more severe. As the child grows older, signs of malnutrition and growth retardation become apparent, with frequent reliance on enemas or other measures to assist with defecation. The most prominent physical finding is abdominal distension, and in some cases, a palpable mass may be detected in the left lower quadrant.
Diagnosis
The diagnosis of congenital megacolon is typically straightforward and is based on the clinical history and presentation. In infants and children, the disease is often characterized by a history of persistent constipation and progressively severe abdominal distension, indicative of chronic partial intestinal obstruction. Several diagnostic tests are used to confirm the diagnosis and assess the extent and location of the lesion:
Imaging Studies
X-ray
Upright abdominal X-rays reveal signs of low-level intestinal obstruction, with dilation of the bowel proximal to the affected segment, as well as gas and fluid accumulation and air-fluid levels. The affected segment itself appears gasless. In neonates, bowel dilation may not be as pronounced as in older children and mainly presents as gaseous distension throughout the intestines.
Barium Enema
This test helps with diagnosis and determination of the length of the affected segment. It is the most commonly used screening tool for congenital megacolon. A typical finding includes a transition zone between the narrowed, spastic distal segment and the dilated proximal segment. Retention of barium observed on follow-up imaging 24 hours later confirms the diagnosis and aids in determining the extent of surgical resection.
Anorectal Manometry
This test is non-invasive and highly valuable for diagnosing congenital megacolon. A lack of the rectoanal inhibitory reflex is a key diagnostic feature.
Biopsy
Histological analysis of tissue samples is the most reliable method for diagnosing congenital megacolon, particularly in difficult cases. Submucosal and muscular biopsies are examined for the presence or absence of ganglion cells and to assess the degree of neural development. The absence of ganglion cells is the definitive pathological criterion for diagnosis.
Treatment
The main treatment for Hirschsprung disease is surgery. For cases where the diagnosis is uncertain or surgery is not immediately feasible, or for preoperative preparation, non-surgical treatments are used to manage symptoms. These include dilation, enemas, and nutritional support to relieve constipation and abdominal distension and to maintain adequate nutrition. For confirmed cases where the patient can tolerate surgery, surgical treatment involves resecting the aganglionic segment and the dilated, hypertrophic proximal colon with degenerative ganglion cells to resolve the functional obstruction. In critically ill patients requiring urgent intervention, a colostomy may be performed first, followed by definitive surgery at a later stage.
In neonates with HD, non-surgical treatments or colostomy are often performed initially, followed by definitive surgery at around six months of age. However, reports of one-stage definitive surgery in neonates have increased in recent years.
Common Surgical Procedures
Swenson Procedure
This involves resecting the megacolon, with the proximal colon pulled through the anus and anastomosed to the anal canal. Because this procedure entails extensive dissection and significant intraoperative bleeding, along with a higher rate of postoperative complications, its use has become less common.
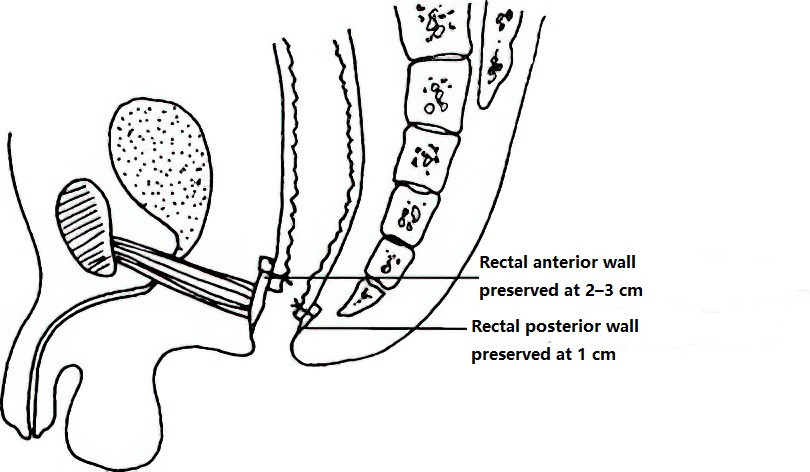
Figure 2 Swenson procedure
Duhamel Procedure
In this technique, the resected proximal colon is brought down and anastomosed to the posterior wall of the rectum after sealing off the blind distal rectal segment.
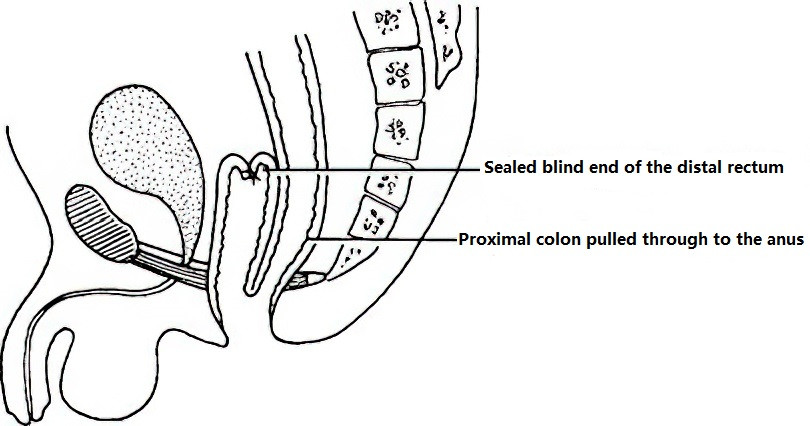
Figure 3 Duhamel procedure
Soave Procedure
This involves mucosal stripping of the rectum, with the colon being pulled through the rectal muscular sheath and sutured to the anal canal mucosa.
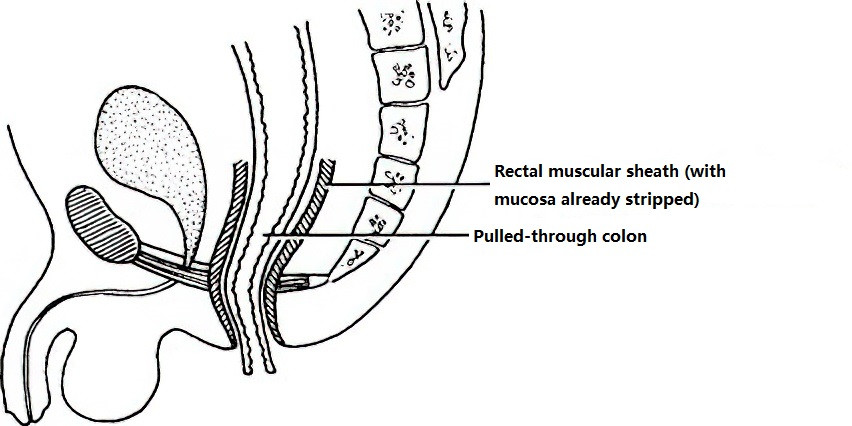
Figure 4 Soave procedure
Laparoscopic-Assisted Hirschsprung Disease Surgery
This minimally invasive procedure facilitates resection of the affected bowel segment, with the normal proximal colon being pulled through and anastomosed to the anal canal. Advantages of laparoscopic-assisted surgery include smaller incisions, faster recovery, and favorable outcomes. Laparoscopic-assisted Soave procedure is currently the most widely adopted surgical technique.