Aortic dissection refers to a pathological condition in which blood enters through a tear in the intimal layer of the aortic wall and infiltrates the media layer, creating a separation between the true and false lumens of the aorta. It typically presents abruptly, progresses rapidly, and carries a high mortality rate.
Etiology and Pathophysiology
The exact cause of aortic dissection remains unclear, but it is commonly associated with factors such as hypertension, genetic connective tissue disorders (e.g., Marfan syndrome, Turner syndrome, Ehlers-Danlos syndrome), inflammatory diseases of the aorta, atherosclerosis and its ulcers, aneurysms, coarctation of the aorta, congenital aortic valve diseases, polycystic kidney disease, advanced age, pregnancy, severe trauma, or iatrogenic injury.
Pathogenesis
Various causative factors result in the destruction or necrosis of the elastic-fiber-rich medial layer of the aortic wall. Fluctuations in blood pressure lead to increased transverse shear stress, causing a tear in the intimal layer. Retrograde or antegrade blood flow propagates the formation of an intramural hematoma, creating a false lumen that communicates with the true lumen (the native lumen of the aorta) through one or multiple openings. This forms the "dissection." Structural abnormalities in the medial layer provide the foundation for the disease, while the presence of the "intimal flap"—a membrane separating the true and false lumens—represents the most characteristic pathological feature of acute aortic dissection.
Approximately 65% of intimal tears originate in the ascending aorta, as it bears the greatest stress. About 25% originate in the descending aorta, often several centimeters distal to the subclavian artery take-off, which is a point of significant blood pressure fluctuation. Another 10% arise from the aortic arch or abdominal aorta. Blood flow through the tear spreads along the long axis of the aortic wall, creating a false lumen that may extend distally (typically) or proximally, involving segments such as the ascending aorta, the aortic arch, or the entire aorta. This can lead to rupture, impaired perfusion of vital organs, and structural damage to the aortic valve and coronary artery ostia, ultimately resulting in aortic valve prolapse, regurgitation, or ischemic myocardial injury.
Clinical studies have shown that acute aortic dissection is often accompanied by systemic inflammatory responses, including leukocytosis, accelerated erythrocyte sedimentation rate, and elevated C-reactive protein levels. Rupture of the dissection may cause acute cardiac tamponade, hemothorax, hemoperitoneum, mediastinal hematoma, or retroperitoneal hematoma.
Mechanisms of Ischemic Complications
Ischemic manifestations associated with aortic dissection may arise through three mechanisms:
- Compression of the true lumen by the false lumen, leading to narrowing of branch artery openings.
- Extension of the dissection into the branch artery walls, causing luminal stenosis.
- Closure of branch artery openings due to the "intimal flap" acting as a mobile valve.
The severity of ischemic complications depends on factors such as the degree of branch artery obstruction, the duration of ischemia, the capacity of collateral circulation, and the tolerance of affected organs or limbs to ischemia.
Classification Based on Timeline
Aortic dissections are classified by time based on symptom duration. Dissections diagnosed within two weeks of symptom onset are classified as acute dissections, those occurring between two weeks and two months as subacute dissections, and those longer than two months as chronic dissections. Chronic dissections are characterized by fibrosis, thickened adherent adventitia, mural thrombus formation, and thrombus organization, often resulting in the development of dissecting aneurysms.
Anatomical Classification
The classification of aortic dissection is based on the location of the intimal tear and the extent of dissection along the aorta. The classification system proposed by DeBakey is as follows:
- Type I: Dissection originates from the ascending aorta and extends to the aortic arch, descending thoracic aorta, or abdominal aorta (or both).
- Type II: Dissection is confined to the ascending aorta.
- Type IIIa: Dissection is limited to the descending thoracic aorta.
- Type IIIb: Dissection involves the descending thoracic aorta and extends to varying degrees into the abdominal aorta.
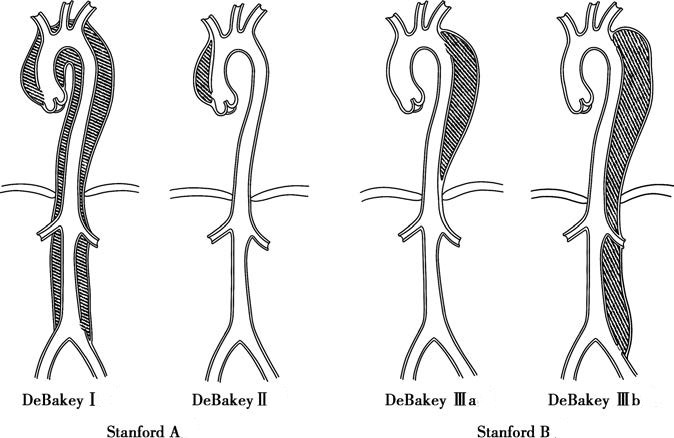
Figure 1 Classification of aortic dissection
The Stanford classification simplifies the anatomical classification criteria to focus solely on the site of the initial tear:
- Stanford Type A: The dissection originates from the ascending aorta (includes DeBakey Type I and Type II).
- Stanford Type B: The dissection originates beyond the left subclavian artery in the descending aorta (includes DeBakey Type IIIa and Type IIIb).
Clinical Manifestations
Acute aortic dissection typically has an abrupt onset, with over 90% of patients presenting with sudden, severe, tearing or knife-like sharp pain in the anterior chest, back, or abdomen. The pain often follows the path of the aorta and may radiate to the abdomen or lower abdomen. Around 80% of patients experience hypertension and tachycardia. Many individuals exhibit restlessness, profuse sweating, and distress. Differential diagnosis includes angina, pulmonary embolism, and myocardial infarction.
As the condition progresses, symptoms and signs related to aortic rupture, aortic regurgitation, and/or impaired perfusion of vital organs may occur:
Symptoms of Aortic Rupture
When the ascending aorta ruptures, blood enters the pericardial cavity, resulting in acute cardiac tamponade and sudden death in most cases within minutes. Thoracic aortic rupture may cause hemothorax, while abdominal aortic rupture may lead to blood accumulation in the retroperitoneal space, manifesting as abdominal pain and distension. Patients with these scenarios often exhibit signs of significant blood loss or even shock.
Symptoms and Signs of Impaired Perfusion
These can vary widely, including angina or myocardial infarction due to coronary blood flow disturbances; syncope, coma, or hemiplegia due to compromised cerebral blood flow when the brachiocephalic trunk is involved; abdominal pain from ischemia of abdominal organs or intestines; acute renal failure due to renal ischemia; the "5 Ps" (pain, pallor, pulselessness, paresthesia, paralysis) due to lower limb ischemia; or paraplegia due to spinal cord ischemia.
Patients with mild aortic regurgitation may present asymptomatically, or symptoms may be overshadowed by pain. Moderate to severe aortic regurgitation can cause palpitations and shortness of breath. In severe cases, acute left heart failure may present with pink, frothy sputum, inability to lie flat, or other symptoms.
Most chronic aortic dissections are asymptomatic before rupture. However, some chronic, expansile aortic dissections may present with chronic pain or symptoms due to compression of adjacent thoracic or abdominal organs. Initial detection often occurs during imaging studies, such as an abnormal chest radiograph or palpable pulsatile abdominal mass. Some patients with chronic aortic dissection may exhibit intermittent claudication, renovascular hypertension, impaired renal function, or abdominal colic due to involvement of aortic branches.
Examination and Diagnosis
Patients with a history of hypertension who present with unexplained, sudden, and severe pain in the chest, back, or abdomen may warrant consideration of this condition. The diagnosis is based on typical clinical manifestations/signs and findings from auxiliary testing.
When aortic dissection is suspected, prompt imaging studies are essential to determine the dissection type, extent of involvement, location of the intimal tear, presence of thrombus in the false lumen, branch vessel and aortic valve involvement, and whether pericardial effusion is present. These findings guide treatment decisions. Whole-aorta CTA is the preferred diagnostic modality and main tool for postoperative follow-up. MRA is also useful for diagnosis but is limited in acute cases due to the longer duration of the procedure. Ultrasound provides high diagnostic accuracy for proximal dissections, but evaluation of the descending aorta is limited. DSA, being an invasive test, is no longer used as an initial diagnostic tool but plays a crucial role in endovascular stent-graft repair. A chest X-ray may reveal signs such as widening of the aortic arch or mediastinum, localized aortic bulges, or massive pleural effusion. ECG and cardiac enzyme testing help differentiate aortic dissection from acute myocardial infarction.
Acute aortic dissection is often misdiagnosed. Beyond differentiation from acute myocardial infarction, other conditions to exclude include acute pericarditis, acute pleuritis, pulmonary embolism, acute abdominal conditions, and acute lower limb arterial embolism.
Treatment
In the acute phase of aortic dissection, sedation, pain relief, continuous ECG monitoring, and supportive therapy play a central role. Blood pressure and heart rate control through medication are used to reduce stress on the aortic wall and prevent further extension or rupture of the dissection.
For Stanford Type A aortic dissections, once diagnosed, emergency surgical intervention is generally the standard approach. This involves thoracotomy and segmental graft replacement under cardiopulmonary bypass.
For acute Stanford Type B aortic dissections, endovascular repair is recommended after stabilization of blood pressure and heart rate with medical therapy. Emergency endovascular repair is indicated for cases where hypertension is medically unmanageable, pain is resistant to treatment, evidence of aortic rupture emerges, or acute ischemia of the lower limbs or kidneys develops. For Type B dissections involving the aortic arch, adjunctive techniques such as branched stents, fenestrated stents, or parallel stents may be considered during endovascular repair.
The clinical success of endovascular repair is defined by complete closure of the rupture site, absence of significant endoleaks or severe complications, and resolution or thrombosis of the false lumen. Compared to open surgery, this method offers advantages such as minimal invasiveness, higher success rates, faster recovery, and fewer complications.