An aortic aneurysm refers to a localized or diffuse dilation or bulging of the aorta that exceeds 1.5 times its normal diameter. This condition arises due to structural damage to the aortic wall caused by various factors and the impact of blood pressure. The aortic wall's middle layer, composed of 45–55 layers of elastic membranes, maintains the aorta's elasticity and tensile strength. During left ventricular systole, part of the kinetic energy generated is stored as potential energy in the aortic wall, which is then converted into forward blood flow during diastole, ensuring effective synchronization between the left ventricle and the aorta. The high blood pressure and shear force within the aorta create a significant risk of rupture in aneurysms, leading to rapid and massive hemorrhage with a high mortality rate.
Classification
Aortic aneurysms are classified based on location into thoracic aortic aneurysms, abdominal aortic aneurysms, and thoracoabdominal aortic aneurysms. Thoracic aneurysms can further involve the aortic root and ascending aorta, the aortic arch, or the descending aorta. Morphologically, aneurysms can be classified into saccular, fusiform, mixed, and dissecting aneurysms. Pathologically, they are divided into true aneurysms, where the aneurysmal wall includes all layers of the arterial structure, and false aneurysms, where the aneurysmal wall consists only of the adventitia, surrounding adhesive tissue, or mural thrombus.
Etiology
The causes of aortic aneurysms include genetic factors, congenital conditions, degenerative changes in aging, inflammatory diseases, autoimmune disorders, hemodynamic factors, infections, and trauma. Aneurysms of the aortic root and ascending aorta are often influenced by genetic factors, typically presenting at a young age, and are frequently associated with bicuspid aortic valve anomalies. However, some ascending aneurysms are sporadic or idiopathic in origin. Descending and abdominal aortic aneurysms are often degenerative and closely associated with atherosclerosis, generally appearing later in life. Conditions such as Marfan syndrome and Behçet's disease, classified as genetic disorders or autoimmune diseases, have a poorer prognosis, with aneurysms or false aneurysms often recurring even after treatment. Traumatic aortic aneurysms, often false or dissecting aneurysms, are strongly linked to high-speed collisions or chest compression injuries and are at high risk of rupture with fatal hemorrhage in a short timeframe. Infectious organisms (e.g., syphilis) may also cause inflammatory damage to the aortic wall, leading to aneurysm formation.
Pathophysiology
The rupture and degradation of elastic fibers and collagen in the vascular wall are pathological changes associated with the formation of aortic aneurysms. Oxidative stress, smooth muscle cell apoptosis, extracellular matrix proteolysis, and adventitial inflammation are significant contributing factors.
According to Laplace's Law, T = P × r (T: tension, P: pressure, r: radius), the tensile force experienced by the aneurysmal wall is directly proportional to arterial blood pressure and aneurysmal radius. Once an aneurysm forms, its enlargement becomes irreversible and progressive. When the tensile force exceeds the elasticity limit of the vessel wall, rupture occurs, leading to massive hemorrhage.
Clinical Manifestations
Symptoms are determined by the size and location of the aneurysm and are often caused by compression, traction, or erosion of surrounding tissues.
Early Stage
Most patients are asymptomatic, with aneurysms often discovered incidentally during imaging studies.
Compression and Pulsatile Masses
Ascending aortic aneurysms may erode the sternum and costal cartilage, forming a visible pulsatile mass on the anterior chest. Such aneurysms may distort the aortic valve annulus, causing leaflet separation and aortic valve insufficiency, leading to murmurs and associated symptoms. Compression of the superior vena cava can result in superior vena cava syndrome, characterized by jugular vein distension and venous engorgement in the face, neck, and shoulders. Compression of the trachea and bronchi can cause cough and dyspnea.
Aneurysms of the aortic arch may compress the trachea and bronchi, causing cough, dyspnea, or lung collapse. Compression of the sympathetic nerve chain may lead to Horner's syndrome. Descending aortic aneurysms may compress the esophagus, causing dysphagia, or compress the recurrent laryngeal nerve, leading to hoarseness (sometimes the initial symptom for patients seeking medical attention). Abdominal aortic aneurysms may compress the gastrointestinal tract, causing upper abdominal fullness and reduced appetite. Compression of the renal pelvis or ureters may produce symptoms of urinary tract obstruction. Compression of the inferior vena cava can cause bilateral deep vein thrombosis of the lower extremities, while bile duct compression may lead to obstructive jaundice.
Embolism
Slow and turbulent blood flow within the aneurysmal lumen increases the risk of thrombus formation. Detachment of mural thrombi can result in embolism of the brain, viscera, or extremities.
Pain and Rupture
Expansion of the aneurysm may cause pain, with persistent dull pain often serving as a precursor to rupture. Sudden, severe pain typically indicates impending rupture. Prognosis is generally poor in such cases, with the most common causes of death being aneurysmal rupture or complications such as aortic-esophageal, aortic-tracheal, or aortic-gastrointestinal fistulas.
Diagnosis and Differential Diagnosis
The diagnosis of an aortic aneurysm primarily relies on imaging studies. X-ray examination often reveals a widened mediastinal opacity and visible calcifications of the aortic wall. CTA (computed tomography angiography) provides precise and intuitive three-dimensional images of the aneurysm, which offer valuable guidance for surgical planning. MRA (magnetic resonance angiography) provides a more detailed depiction of the vessel wall contrast. Coronal and sagittal scans allow imaging of the aneurysm and longitudinal cross-sections of the lumen. However, MRA is costly, time-consuming, and poses risks for hemodynamically unstable patients. Ultrasound enables visualization of aortic aneurysms and intravascular lesions while assessing cardiac structures. It is suitable for rapid evaluation in hemodynamically unstable patients and perioperative monitoring. Given advances in non-invasive imaging techniques, thoracic aortography is now rarely used alone for diagnosing thoracic aortic aneurysms. Differential diagnosis should distinguish aneurysms from aortic dissection (especially chronic dissection with an enlarged false lumen mimicking an aneurysm), mediastinal tumors, central lung cancer, abdominal masses, and other conditions.
Treatment and Prognosis
Currently, there are no specific pharmacological treatments for aortic aneurysms. In cases of early detection, strict control of blood pressure and heart rate is recommended alongside regular follow-ups to monitor disease progression. For aneurysms diagnosed with clear surgical indications, treatment options include conventional open surgery, endovascular repair, or hybrid procedures.
Surgical indications include:
- Development of compression symptoms, rupture, or symptoms of impending rupture.
- Aneurysm diameter exceeding 5 cm.
- Aneurysm growth exceeding 1 cm per year.
- Early treatment is required for false aneurysms and dissecting aneurysms.
Surgical contraindications include:
- Damage or dysfunction of vital organs (e.g., heart, brain, liver, kidneys).
- Poor systemic condition rendering treatment intolerable.
Conventional Open Surgery
Open surgery involves the replacement of the affected aortic segment with an artificial graft. The surgical approach and postoperative outcomes depend on the anatomical location of the aneurysm and may require techniques such as cardiopulmonary bypass, deep hypothermic circulatory arrest, or selective cerebral perfusion for support.
The surgical mortality rate ranges from 4% to 10%. Major complications include hemorrhage, severe arrhythmias, coronary insufficiency, central nervous system complications, chylothorax, gastrointestinal and urinary system injuries, as well as cardiac, pulmonary, and renal failure.
Common open surgical procedures include:
- Bentall Procedure (composite repair of the ascending aorta, aortic valve replacement, and coronary artery reimplantation): This procedure addresses aneurysms of the aortic root causing aortic valve annulus enlargement and valve insufficiency. It is commonly performed in Marfan syndrome patients.
- Aortic Arch Replacement: This is employed for isolated aortic arch aneurysms. The innominate artery, left common carotid artery, and left subclavian artery are mobilized and reconstructed using a four-branch artificial graft. Cardiopulmonary bypass, combined with deep hypothermic circulatory arrest and antegrade or retrograde perfusion, is generally required to protect the brain.
- Descending Aortic Replacement: This involves addressing descending aortic aneurysms, which may compromise the blood supply to the spinal cord and abdominal organs. Techniques such as left-heart bypass or partial cardiopulmonary bypass using femoral artery-to-vein circulation may help protect the spinal cord and kidneys.
- Abdominal Aortic Replacement: Used for infrarenal abdominal aortic aneurysms and does not require cardiopulmonary bypass. The abdominal aorta and bilateral common, external, and internal iliac arteries are mobilized, and a bifurcated artificial graft is used to reconstruct the abdominal aorta and iliac arteries.
- Thoracoabdominal Aortic Replacement with Visceral Artery Reconstruction: This addresses aneurysms involving both thoracic and abdominal segments, including those extending to renal arteries, superior mesenteric arteries, and celiac trunk. The procedure is lengthy and highly invasive. The Crawford method is commonly employed, although it has a perioperative mortality rate of 5%–10%. Complications such as ischemia of the kidneys, lungs, and spinal cord remain challenges despite the use of techniques to minimize risks.
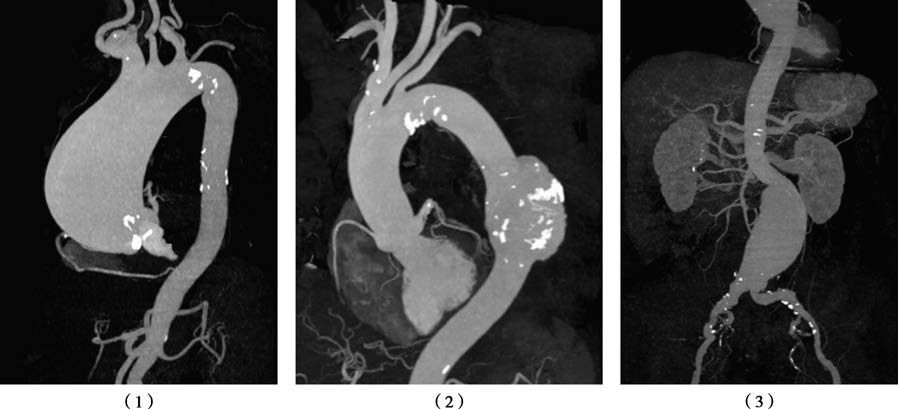
Figure 1 Aortic aneurysm diagnosis via CTA
1, Three-dimensional reconstruction of an ascending aortic aneurysm.
2, Three-dimensional reconstruction of a descending aortic aneurysm.
3, Three-dimensional reconstruction of an abdominal aortic aneurysm.
Endovascular Aneurysm Repair (EVAR)
Endovascular repair avoids thoracotomy or laparotomy and cardiopulmonary bypass. A membrane-covered stent is placed within the aortic lumen to exclude the aneurysmal cavity. This approach is associated with smaller surgical trauma, faster recovery, fewer complications, and broader suitability, particularly for descending aortic aneurysms, abdominal aortic aneurysms, and some aneurysms involving the aortic arch.
Advances in endovascular devices now allow treatment of thoracic aortic aneurysms involving supra-aortic branches, using techniques such as fenestrated stents, branched stents, parallel stents, or single-piece trifurcated stents. While there are no absolute contraindications for thoracic endovascular aortic repair (TEVAR), careful preoperative assessment is essential, particularly for complex reconstructions involving double or triple supra-aortic branches. For facilities without the capability to perform such complex reconstructions, conventional open surgery is preferred. Pulmonary endovascular repair carries a surgical mortality rate of 2%–6.2%. Complications include endoleaks and stent graft migration. As endovascular techniques continue to develop and gain popularity globally, many centers have adopted fully endovascular approaches for the repair of aortic aneurysms.
Hybrid Procedures
Hybrid surgery combines conventional open surgical techniques with endovascular repair, using both artificial grafts and stent grafts to manage aortic aneurysms. Hybrid "one-stop" surgery often requires multifunctional operating theaters equipped with extracorporeal circulation devices and digital subtraction angiography (DSA).