Tetralogy of Fallot (TOF) is a cardiac anomaly characterized by right ventricular outflow tract or conal developmental abnormalities. It involves four pathological anatomical features: pulmonary stenosis, ventricular septal defect, overriding aorta, and right ventricular hypertrophy. Pulmonary stenosis can occur at various sites, including the body and infundibulum of the right ventricle, pulmonary valve and annulus, main pulmonary artery, as well as the left and right pulmonary arteries. The narrowing may involve a single site or multiple locations. With age, progressive hypertrophy, fibrosis, and endocardial thickening of the right ventricular muscle bundles exacerbate the obstruction of the right ventricular outflow tract. Right ventricular hypertrophy is secondary to pulmonary stenosis. Common associated anomalies in tetralogy of Fallot include atrial septal defect, right-sided aortic arch, patent ductus arteriosus, and persistent left superior vena cava.
Pathophysiology
Pulmonary stenosis and ventricular septal defect form the basis of the pathological and physiological changes in tetralogy of Fallot, manifested in four key aspects:
The systolic pressure peaks of both the left and right ventricles become equal. The right ventricular pressure cannot exceed systemic pressure, which protects right ventricular function from progressive pressure overload, explaining why congestive heart failure is rare in clinical settings. In adults with tetralogy of Fallot, pressure overload in the right ventricle may arise from left ventricular hypertension, leading to right ventricular myocardial hypertrophy and frequently tricuspid regurgitation.
The direction of intracardiac shunting depends primarily on the severity of the obstruction in the right ventricular outflow tract and systemic vascular resistance. Tetralogy of Fallot is generally characterized by a right-to-left shunt, but a sudden drop in systemic resistance or intense contraction of the right ventricular infundibulum may sharply reduce pulmonary blood flow, leading to cyanotic spells. During squatting, systemic resistance increases, decreasing the right-to-left shunting, alleviating cyanosis, and easing hypoxic symptoms.
The degree of pulmonary blood flow reduction is primarily determined by the severity of pulmonary stenosis rather than the location of the narrowing.
Chronic hypoxia results in polycythemia and the development of collateral vessels between systemic and pulmonary circulation.
Clinical Manifestations
Most patients exhibit dyspnea from birth, progressing to cyanosis between 3 to 6 months of age, which worsens with time. Hypoxia leads to reduced exercise tolerance and physical development delayed compared to peers. Feeding difficulties and growth retardation are common. Squatting is a characteristic posture frequently seen in children, as it helps relieve cyanosis and dyspnea. Hypoxic spells, often seen in infants with isolated infundibular stenosis, typically occur in the morning or after exertion. These spells manifest as sudden dyspnea, worsened cyanosis, and may progress to syncope, seizures, or even death.
Physical Examination: Patients often show signs of growth retardation, cyanosis of the lips, conjunctiva, and extremities, as well as clubbing of fingers and toes. A grade II–III systolic ejection murmur can typically be heard along the left sternal border at the 2nd to 4th intercostal space, along with a weakened or absent second heart sound in the pulmonary valve area. In cases of severe pulmonary stenosis, the murmur becomes faint or inaudible.
Ancillary Examinations
Electrocardiogram (ECG)
Findings may include right-axis deviation and right ventricular hypertrophy.
Chest X-Ray
The heart size is normal or slightly enlarged, with reduced pulmonary vascular markings. The pulmonary artery segment may appear concave, the cardiac apex rounded and elevated, forming a "boot-shaped heart." The ascending aorta may appear widened.
Ultrasound
Findings include stenosis of the right ventricular outflow tract, pulmonary valve, or main pulmonary artery. Right ventricular enlargement and hypertrophied right ventricular walls may be present, along with interruption of ventricular septal continuity. The diameter of the ascending aorta may be increased, with the aorta overriding the ventricular septum. A right-to-left shunting signal at the ventricular septal level can be observed.
Laboratory Tests
Elevated red blood cell count, hematocrit, and hemoglobin levels are common and correlate with the severity of cyanosis. Arterial oxygen saturation is reduced. In patients with severe cyanosis, platelet counts and fibrinogen levels decrease significantly, while platelet function is impaired, leading to prolonged clotting and prothrombin times.
Diagnosis
Diagnosis of tetralogy of Fallot can be made based on symptoms, physical findings, and the results of the above examinations. CT angiography (CTA) provides detailed information on the development of the left and right pulmonary arteries. Right heart catheterization may reveal elevated right ventricular pressure, low pulmonary artery pressure, and equal systolic pressures in the right ventricle, left ventricle, and aorta. Cardiovascular angiography enables a clear visualization of the relationship between the aorta and pulmonary artery, the site and severity of pulmonary stenosis, the branching of the pulmonary arteries, left ventricular development, and systemic-pulmonary collateral vessels. Tetralogy of Fallot may be complicated by cerebral thrombosis, cerebral abscess, infective endocarditis, or hypertension.
Treatment
Surgical Indications
Symptomatic newborns and infants should undergo surgical intervention at the earliest feasible time, with one-stage complete repair being implemented when appropriate. For asymptomatic patients or those with mild symptoms, current recommendations favor elective complete repair around six months of age to reduce secondary myocardial and systemic damage. Two essential criteria for complete repair include:
- Normal development of the left ventricle, with left ventricular end-diastolic volume index >30 ml/m2
- Adequate pulmonary artery development, with McGoon ratio ≥1.2 or Nakata index ≥150 mm2/m2 (McGoon ratio is defined as the sum of the diameters of the right and left pulmonary arteries divided by the diameter of the descending aorta at the diaphragm level, with a normal value >2.0; Nakata index is defined as the sum of the cross-sectional areas of the right and left pulmonary arteries divided by body surface area, with a normal value ≥330 mm2/m2)
For patients who do not meet these criteria or those with coronary artery anomalies that impede right ventricular outflow tract reconstruction, palliative surgery is considered necessary. Both palliative and complete repair surgical approaches share contraindications in cases of refractory heart failure or severe hepatic and renal dysfunction.
Surgical Techniques
Palliative Surgery
The purpose of palliative surgery is to increase pulmonary blood flow, enhance arterial oxygen saturation, and promote the development of the left ventricle and pulmonary vasculature, thereby creating conditions suitable for complete repair in the future. Several surgical techniques are available, and the two most commonly used are:
- Systemic-to-pulmonary shunt surgery: The classic approach is the modified Blalock-Taussig shunt, performed without cardiopulmonary bypass using a 4–5 mm artificial vascular graft to connect the left subclavian artery to the left pulmonary artery, or the innominate artery to the right pulmonary artery.
- Right ventricular outflow tract reconstruction: Conducted under cardiopulmonary bypass, this involves longitudinal incision through the right ventricle and pulmonary artery without closure of the ventricular septal defect. Hypertrophic muscle from the infundibulum of the right ventricle is excised, and the right ventricular outflow tract and pulmonary artery are widened using patches made of autologous pericardium or artificial materials.
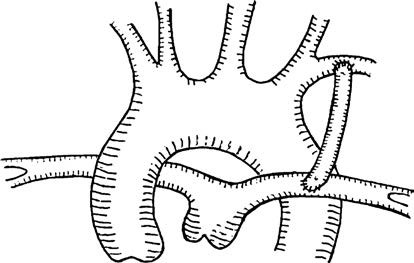
Figure 1 Modified Blalock-Taussig shunt
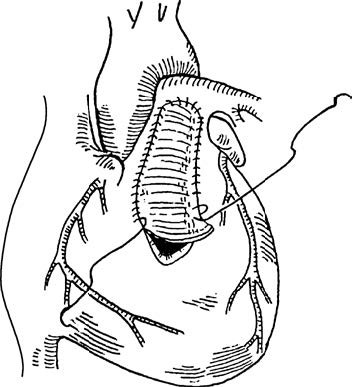
Figure 2 Right ventricular outflow tract patch across pulmonary valve annulus
Patients who have undergone palliative surgery require close follow-up, and complete repair should be considered once the conditions are met. Common complications of palliative surgery include excessive shunt flow leading to congestive heart failure and pulmonary edema, insufficient shunt flow resulting in inadequate improvement of cyanosis, chylothorax, Horner syndrome, and infective endocarditis.
Complete Repair Surgery
Under cardiopulmonary bypass, access to the heart is gained through incisions in the right atrium or right ventricle. Hypertrophied muscle bundles of the ventricular wall and septum are excised to reconstruct the right ventricular outflow tract. The ventricular septal defect is closed with a patch, redirecting the overriding aorta to the left ventricle. Autologous pericardium or artificial graft material is used to widen the right ventricular outflow tract, pulmonary valve annulus, main pulmonary artery, and branch pulmonary arteries as needed.
Common complications of complete repair surgery include low cardiac output syndrome, perfusion-related pulmonary issues, residual ventricular septal defects, and third-degree atrioventricular block.