A ventricular septal defect (VSD) is an abnormal communication between the ventricles caused by incomplete development of the ventricular septum during the fetal period. Based on the location of the defect, VSDs can be classified into three main types: perimembranous, infundibular (outlet), and muscular, along with several subtypes. Perimembranous defects are the most common, followed by infundibular defects, while muscular defects are relatively rare. The majority of VSDs are solitary, although muscular defects can occasionally present as multiple.
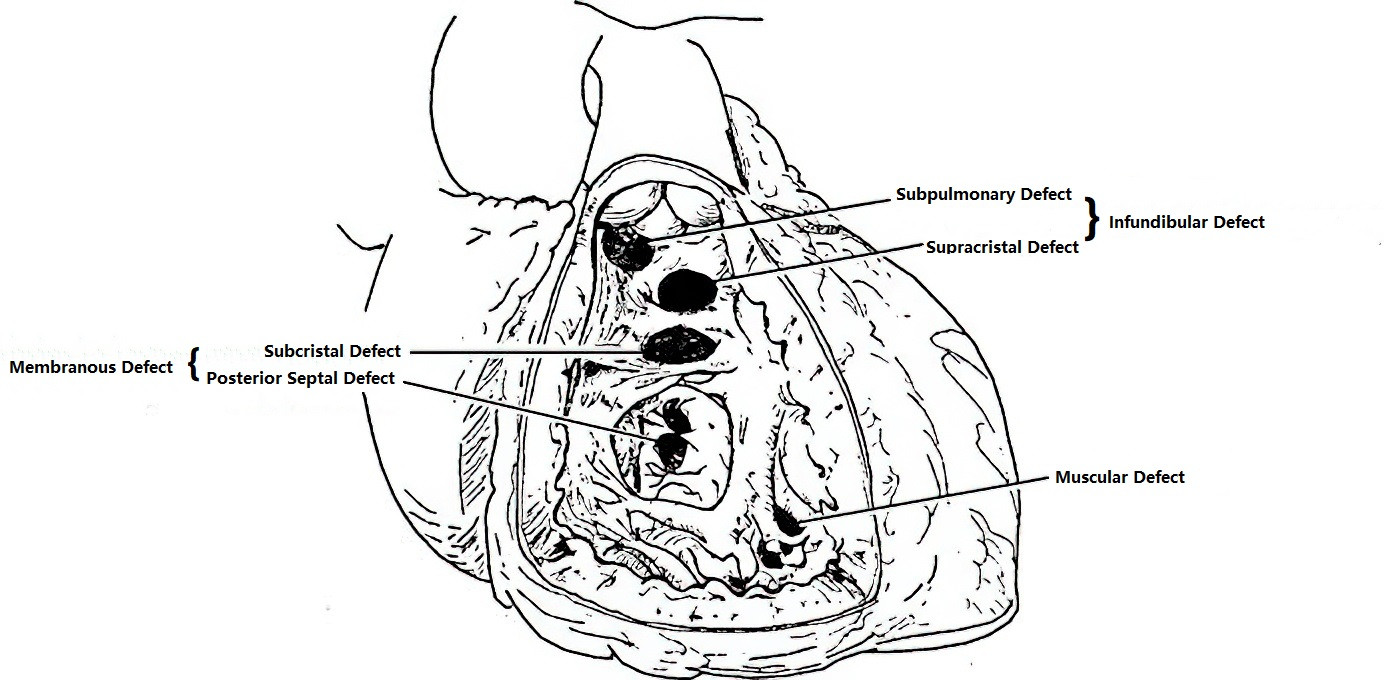
Figure 1 Illustration of VSD types
Pathophysiology
The hemodynamic changes in VSD depend primarily on the size of the defect, the pressure gradient between the left and right ventricles, and the pulmonary vascular resistance. Small defects result in minimal shunting and have limited impact on cardiac function, but they significantly increase the risk of infective endocarditis. Large defects allow greater shunting, which increases pulmonary circulation and imposes a volume load on the left ventricle, leading to enlargement of the left atrium and left ventricle. Increased pulmonary blood flow can cause pulmonary arteriole spasm and elevated pulmonary artery pressure early in the disease course. This leads to increased right ventricular afterload and subsequent right ventricular hypertrophy. Over time, progressive pulmonary vascular changes result in pulmonary arterial hypertension (PAH). With advanced PAH, the shunting reverses to right-to-left, resulting in Eisenmenger syndrome.
Clinical Presentation
Patients with small defects and minimal shunting are often asymptomatic. Large shunts may manifest shortly after birth with recurrent respiratory infections, congestive heart failure, feeding difficulties, and growth retardation. Children who survive infancy with large defects often develop reduced exercise tolerance, dyspnea, and palpitations with exertion. Progressive cyanosis and right-sided heart failure may ultimately occur. Patients with VSD are predisposed to developing infective endocarditis.
Auscultation in patients typically reveals a grade III or higher harsh and loud holosystolic murmur heard at the second to fourth intercostal spaces along the left sternal border, often accompanied by a systolic thrill. The murmur's location correlates with the anatomical position of the VSD. In large shunts, a soft diastolic murmur at the cardiac apex may indicate relative mitral valve stenosis. In cases of pulmonary hypertension, murmurs in the precordial area become softer, shorter, and less intense, and the second heart sound at the pulmonary valve is accentuated. A diastolic murmur of pulmonary regurgitation may also develop.
Auxiliary Investigations
Electrocardiography (ECG)
Small defects are typically associated with normal ECG findings. Larger defects may show left ventricular hypertrophy. Pulmonary hypertension can lead to findings of biventricular hypertrophy or right ventricular hypertrophy with strain patterns.
Chest X-ray
In cases of small defects, pulmonary congestion and changes in cardiac silhouette are minimal. Larger defects are associated with left ventricular enlargement, prominence of the pulmonary artery segment, and increased pulmonary vascular markings. With obstructive pulmonary hypertension, the degree of enlargement of the left and right ventricles may paradoxically decrease, and a "pruned tree" appearance of pulmonary vasculature may be observed.
Echocardiography
Ultrasound provides detailed visualization of the size, location, and flow direction of the defect, along with any associated malformations. It also offers preliminary insights into pulmonary artery pressure. VSD is often associated with enlargement of the left atrium, left ventricle, or both ventricles.
Diagnosis
The diagnosis is generally based on the location and characteristics of the murmur, in combination with findings from echocardiography and chest X-ray. Severe pulmonary hypertension may necessitate right heart catheterization to measure pulmonary artery pressure and calculate pulmonary vascular resistance to determine surgical indications.
Treatment
Indications for Surgery
The decision for surgery is based on a comprehensive assessment of symptoms, signs, cardiac function, defect size and location, severity of pulmonary hypertension, and the degree of atrial and ventricular dilation. Age and body weight are not considered primary determinants for surgical intervention.
Large Ventricular Septal Defects (VSDs) (defect diameter greater than two-thirds of the aortic annulus diameter)
Neonates or infants presenting with feeding difficulties, recurrent pulmonary infections, or congestive heart failure are candidates for urgent surgical intervention. For older children and adults with a pulmonary-to-systemic blood flow ratio greater than 2, significant cardiac murmurs, pulmonary congestion on X-ray, and echocardiographic evidence of predominant left-to-right shunting, proactive surgery is recommended.
Moderate VSDs (defect diameter between one-third and two-thirds of the aortic annulus diameter)
Surgical intervention is indicated in cases of recurrent pulmonary infections, growth retardation, as well as associated cardiac enlargement, pulmonary congestion, and pulmonary hypertension.
Small VSDs (defect diameter less than one-third of the aortic annulus diameter)
Close observation is appropriate, as approximately half of the VSDs spontaneously close by the age of three, especially membranous defects. For patients showing signs of cardiac enlargement, pulmonary congestion on echocardiography or X-ray, or complications such as infective endocarditis, surgical intervention is strongly recommended.
Special Situations
Subpulmonary (outlet) defects, which are prone to complications such as aortic valve prolapse and resulting aortic regurgitation, should be treated surgically as early as possible. Eisenmenger syndrome is considered a contraindication for surgery.
Surgical Techniques
Open-heart surgery under direct visualization remains the primary approach for treating VSDs and may involve minimally invasive approaches such as total thoracoscopic surgery or thoracoscopic-assisted small incisions through the right axilla or right anterolateral chest. After establishing cardiopulmonary bypass, the defect is exposed via an incision in the right atrium, right ventricle, or pulmonary artery, depending on its location. Small defects are closed with direct sutures, while larger defects require repair using an autologous pericardial patch or synthetic patch material. Care must be taken intraoperatively to avoid damage to the aortic valve and atrioventricular conduction pathways.
Transcatheter and Periventricular Device Closure
These methods, performed under guidance from fluoroscopy or transesophageal echocardiography, offer the advantages of reduced invasiveness and quicker recovery. However, these techniques are only suitable for patients with appropriately sized and positioned defects. Common complications include valvular insufficiency and complete atrioventricular block (third-degree AV block).