Penetrating ocular injury involves a full-thickness rupture of the ocular wall caused by the stabbing or cutting action of sharp objects. It represents a "from outside to inside" mechanism of injury, potentially accompanied by intraocular damage or prolapse of intraocular tissues. Common causes include injuries from knives, needles, and scissors. The prognosis depends on factors such as the location, extent, and severity of the injury, the presence or absence of complications like infection, and the timeliness and appropriateness of treatment.
Clinical Manifestations
Based on the location of the wound, penetrating ocular injuries can be categorized into three types:
Corneal Penetrating Injury
This is more common and is classified into simple and complex types:
- Simple type: The corneal wound is relatively small, regular, and often self-sealing, with no iris incarceration.
- Complex type: The wound is larger and irregular, commonly associated with iris prolapse and incarceration, shallow anterior chamber, and possibly lens rupture, cataract formation, or posterior segment damage. Symptoms include significant ocular pain, tearing, and reduced visual acuity.
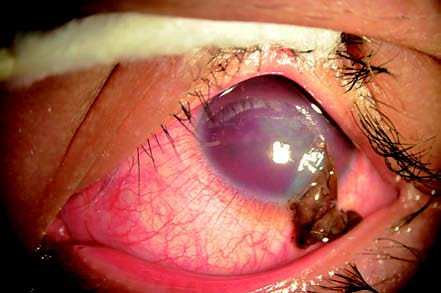
Figure 1 Corneal penetrating injury
A wound is visible on the left cornea, accompanied by iris prolapse and incarceration, as well as hyphema in the anterior chamber.
Corneoscleral Penetrating Injury
This type involves wounds affecting both the cornea and sclera. It may result in damage or prolapse of the iris, ciliary body, lens, and vitreous, along with intraocular hemorrhage. Symptoms include pronounced ocular pain, irritation, and marked vision loss.
Scleral Penetrating Injury
Smaller scleral wounds may be overlooked, with only subconjunctival hemorrhage visible on the surface. Larger scleral wounds are often accompanied by damage to the choroid, vitreous, and retina, as well as hemorrhage, leading to a poor prognosis.
Treatment
Immediate protective dressing of the injured eye is essential, followed by emergency ophthalmic consultation. For complex cases, a two-stage surgical approach is often adopted:
- Primary repair: Restoring the integrity of the globe.
- Prevention and management of complications, such as infection.
- Secondary surgery: Performed when necessary.
Primary Wound Management
Simple Corneal Wounds
Small wounds with good apposition and an intact anterior chamber may not require suturing, and protective bandaging is sufficient. Wounds larger than 3 mm, which often exhibit partial closure or poor alignment causing corneal irregularities, typically require meticulous microsurgical suturing to reconstruct the anterior chamber. The use of a contact lens may help reduce irregular astigmatism following the injury.
Complex Corneal Wounds
For incarcerated iris, intraocular repositioning can be attempted using an antibiotic solution. If repositioning is not possible (due to significant damage, ischemia, contamination, or injuries occurring more than 24 hours prior), excision may be necessary. Corneal wounds should be sutured carefully.
Corneoscleral Wounds
These should be managed by first suturing at the corneal limbus, then closing the corneal and scleral defects sequentially. Dislocated ciliary body and retina should be repositioned, while prolapsed lens and vitreous may require removal.
Scleral Wounds
Suturing proceeds from anterior to posterior with gradual exposure of the wound. Temporary disinsertion of extraocular muscles may be necessary. The exit wound of a perforating injury is generally left to self-seal and is not sutured.
Post-Trauma Inflammation and Infection Control
Administration of tetanus prophylaxis is standard, along with systemic antibiotics and corticosteroids. Frequent use of topical antibiotic eye drops and mydriatic agents is recommended.
Secondary Surgery
Depending on the degree of intraocular structural damage, secondary surgical interventions, such as intraocular or vitreoretinal surgeries, are often performed 1 to 2 weeks after the initial injury. These procedures may address traumatic cataracts, vitreous hemorrhage, or retinal detachment.
Complications and Management
Traumatic Infectious Endophthalmitis
Traumatic infectious endophthalmitis is a severe complication of ocular trauma. The incidence of endophthalmitis after open-globe injury without intraocular foreign bodies ranges from 3.1% to 11.9%, and the rate increases significantly when intraocular foreign bodies are present, ranging from 3.8% to 48.1%. The pathogens causing traumatic endophthalmitis are not entirely the same as those in other forms of endophthalmitis, such as postoperative endophthalmitis. Gram-positive bacteria (e.g., Staphylococcus species) are the most common pathogens, followed by Gram-negative bacteria (e.g., Pseudomonas species), while fungal endophthalmitis is relatively rare. Risk factors for endophthalmitis include the type of injury, the presence of retained intraocular foreign bodies, timeliness and adequacy of post-injury treatment (e.g., wound closure and appropriate medication), and pre-existing medical conditions. The condition progresses rapidly, with severe ocular and headache pain, prominent irritation symptoms, dramatic vision loss, and even loss of light perception. Signs include marked conjunctival edema and hyperemia, corneal clouding, fibrinous exudate or hypopyon in the anterior chamber, and vitreous opacities or abscess formation. Severe cases may lead to corneal-scleral necrosis, perforation, or even orbital cellulitis.

Figure 2 Traumatic infectious endophthalmitis
The right eye displays mixed conjunctival hyperemia and edema, corneal necrosis near the margin, central corneal opacity, and suspected hypopyon in the anterior chamber. Intraocular details are obscured.
Management
Endophthalmitis requires immediate treatment. Adequate mydriasis is essential, along with high-dose antibiotics and corticosteroids administered locally and systemically. Intravitreal injections provide effective drug concentrations, with 1 mg of vancomycin, 2 mg of ceftazidime (if no contraindications such as drug allergies exist), and 0.4 mg of dexamethasone commonly used. Before injection, aqueous humor and vitreous samples should be collected for bacterial culture and sensitivity testing, and the medication regimen should be adjusted based on the results. In severe infections, emergency vitrectomy combined with intravitreal drug infusion may be necessary. For cases with poor inflammation control, treatment may be repeated within 48–72 hours. Delayed intervention (e.g., overnight delays) could result in the inability to preserve the globe.
Sympathetic Ophthalmia
Sympathetic ophthalmia is a bilateral granulomatous uveitis that occurs following an open-globe injury or intraocular surgery, caused by exposure of intraocular antigens leading to an autoimmune response. The incidence is approximately 0.2% after trauma and about 0.07‰ after intraocular surgery. The condition primarily involves cellular immunity, and the antigens likely originate from melanin, retinal pigment epithelium, or photoreceptor outer segments. Infections may play a role in antigen activation.
Clinical Manifestations
The condition can develop anywhere from 5 days to several months or even years after trauma or surgery, although it most commonly appears 2 weeks to 2 months post-injury. The onset is usually insidious, presenting as granulomatous inflammation. It may manifest as anterior uveitis, posterior uveitis, intermediate uveitis, or panuveitis, with panuveitis being the most frequent form. The injured eye (referred to as the "exciting eye") exhibits persistent and worsening uveitis symptoms, including keratic precipitates (KPs) and small pearl-like grayish nodules at the pupillary margin. After a latent period, the unaffected eye (referred to as the "sympathizing eye") develops similar uveitis, with sudden and profound visual loss.
Ophthalmoscopic examination may reveal yellowish-white, punctate lesions in the retina, often in the peripheral areas (termed Dalen-Fuchs nodules). Sympathetic ophthalmia typically follows a chronic, relapsing course. In the late stages, widespread retinal pigment epithelial atrophy can result in a reddish appearance of the fundus, resembling the "sunset glow fundus" observed in Vogt-Koyanagi-Harada disease. Inadequate treatment or poor disease control may lead to secondary glaucoma, retinal detachment, or phthisis bulbi. Extraocular manifestations, such as vitiligo, depigmentation of the hair, alopecia, hearing loss, or meningeal irritation, may also occur.
Management
Early wound closure, repositioning or removal of prolapsed uveal tissue, and infection prevention could potentially reduce the risk of developing this condition. Once diagnosed, the condition should be managed with corticosteroids and mydriatic agents as appropriate for uveitis. Topical corticosteroids and cycloplegics may be used for anterior uveitis, while systemic corticosteroids are preferred for posterior or panuveitis. Immunosuppressive agents may be considered in refractory cases. With proper treatment, many patients recover some level of visual function. Removing the injured (exciting) eye does not always halt disease progression, and in some cases, careful treatment of the exciting eye can restore vision. The effectiveness of eye removal in prevention remains debatable, as does the choice between enucleation and evisceration. From a cosmetic perspective, the latter is more commonly favored.
Traumatic Proliferative Vitreoretinopathy
Excessive reparative responses at the wound site or within the eye may lead to fibrous tissue proliferation, causing tractional retinal detachment. Vitrectomy may be performed at an appropriate stage, although some affected eyes may ultimately become atrophic.
"Traumatic retinal detachment" is a broader term and can result from various mechanisms, including retinal tears, subretinal hemorrhage, exudation, or traction (e.g., retinal incarceration or wound-related fibrous tissue proliferation), either individually or in combination. Poor prognosis is expected with concurrent macular damage, large retinal tears, or severe tractional detachment, often necessitating vitrectomy. In cases with extensive corneal lacerations, temporary artificial corneas or endoscopic-assisted surgery may be utilized during the procedure.