Iris and Pupil Abnormalities
Different degrees and locations of injury result in various manifestations:
- Tear of the pupillary margin and sphincter rupture, characterized by irregular tears or radial splits in the iris stroma.
- Iridodialysis, where the iris root appears as a crescent-shaped defect. The pupil takes on a "D" shape, which may cause monocular diplopia. In cases of complete iris detachment, it is referred to as traumatic aniridia.
- Traumatic mydriasis due to damage to the sphincter muscle of the pupil, typically presenting as moderate dilation with an irregular and poorly reactive pupil.
- Ciliary muscle or nerve damage, potentially resulting in accommodative paralysis and difficulty with near vision.
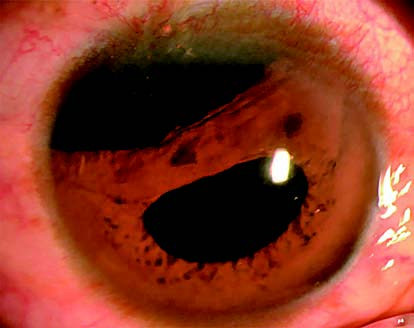
Figure 1 Iridodialysis
A crescent-shaped detachment of the iris root in the superotemporal quadrant of the right eye, resulting in a "D"-shaped pupil.
Treatment usually involves no special intervention for tears in the pupillary margin or stroma. Iridodialysis with diplopia symptoms may be addressed with iris suture surgery. Mild traumatic mydriasis may recover partially or fully, but severe cases may not recover. For accompanying accommodative paralysis, corrective lenses may be used to address near vision impairment.
Hyphema
Hyphema primarily results from iris vessel rupture. Minimal bleeding may only be visible as red blood cells in the aqueous humor. More significant bleeding can pool in the anterior chamber, creating a fluid level. Based on the volume of hyphema relative to the anterior chamber, it is classified into three grades: Grade I involves less than one-third, Grade II one-third to two-thirds, and Grade III more than two-thirds. The actual height of the blood level (in mm) may also be documented. Severe cases can fill the anterior chamber completely with blood, making it appear black. Most hyphema cases resolve spontaneously. However, extensive bleeding or rebleeding during resorption (occurring in 16–20% of cases, typically 2–3 days post-injury) may lead to secondary glaucoma. Corneal endothelial damage, elevated intraocular pressure, and extensive bleeding may cause corneal blood staining, where the stroma becomes brownish-yellow with a central discoid haze that later transitions to a yellow-white color, often resolving very slowly (over approximately one year). Severe cases may result in permanent corneal opacity.
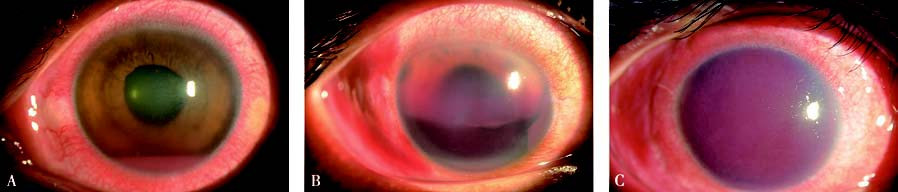
Figure 2 Hyphema
(A) Grade I hyphema.
(B) Grade II hyphema.
(C) Grade III hyphema.
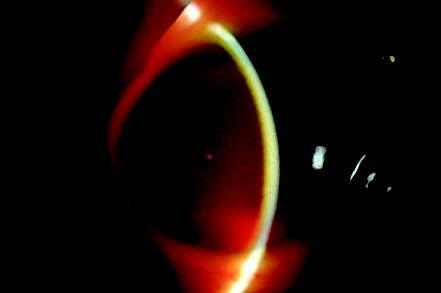
Figure 3 Corneal blood staining
Slit-lamp microscopy shows disc-like central stromal haze and brownish-yellow discoloration of the corneal stroma alongside anterior chamber hemorrhage, with unclear visualization of intraocular structures.
Treatment involves:
- Bed rest in a semi-recumbent position with sedatives and bilateral eye patching to immobilize the eyes.
- Topical corticosteroid eye drops for five days.
- Mydriatic agents delayed for five days due to potential rebleeding risks.
- Ocular hypotensive agents if intraocular pressure rises.
- Daily monitoring of hyphema absorption
When there is a significant amount of hyphema with slow resorption, particularly accompanied by dark-colored blood clots and elevated intraocular pressure, surgical intervention such as anterior chamber irrigation or clot excision should be considered if pharmacological treatment fails to control intraocular pressure within 5 to 7 days. This approach aims to prevent corneal blood staining and optic nerve damage.
Traumatic Glaucoma
Glaucoma secondary to ocular trauma may arise from multiple causes, including blunt or open globe injuries, chemical damage, and electromagnetic radiation. Blunt contusions are the most common cause. The angle of the anterior chamber may remain open or become closed. Elevated intraocular pressure may manifest within days after injury or years later. The condition can present as transient intraocular pressure fluctuations or secondary glaucoma requiring medical or surgical intervention. Blunt trauma-induced glaucoma may result from iris and ciliary body inflammation, angle recession (caused by separation of circular and longitudinal fibers of the ciliary muscle, with posterior displacement of the iris root and widening of the anterior chamber angle), lens dislocation, or intraocular hemorrhage.
Traumatic Hypotony
This condition is often caused by ciliary body separation and presents with visual decline, image distortion, shallow anterior chamber, optic disc edema, retinal vein dilation, macular edema, and stellate folds. The axial length shortens, and hyperopic correction may improve vision slightly. Chronic hypotony can lead to permanent macular and optic nerve functional damage.
Treatment may include initial application of 1% atropine for mydriasis and oral prednisone. Some cases may recover gradually. If medical treatment fails, surgical procedures such as ciliary body suturing may be considered, while monitoring for complications like surgical bleeding and postoperative elevated intraocular pressure.