Etiology and Pathogenesis
When the eye is in a relaxed state of accommodation, parallel light rays from a distant source (typically considered beyond 5 meters) are refracted by the eye's optical system and focused in front of the retina rather than on it, resulting in an unclear visual image on the retina. This refractive state is referred to as myopia.
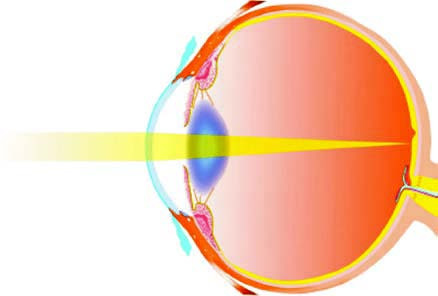
Figure 1 Myopic eye
The pathogenesis of myopia has not been fully elucidated, but several theories have been proposed, including the accommodation lag theory, peripheral defocus theory, neurochemical-related theory, and scleral hypoxia theory. Generally, the development and progression of myopia are believed to result from a combination of genetic and environmental factors.
Genetic Factors
Myopia is associated with autosomal dominant inheritance, autosomal recessive inheritance, and X-linked inheritance. Studies have shown that children of parents who both have myopia have a significantly higher prevalence of myopia. Monozygotic twins exhibit greater concordance in refractive status compared to dizygotic twins. Genetic factors are considered to play a role in the development of high myopia. Numerous studies have identified genetic loci associated with high myopia, suggesting a strong relationship between high myopia and genetic predisposition.
Environmental Factors
Prolonged Near Work
Epidemiological studies reveal that the prevalence of myopia is significantly higher among individuals engaging in extended periods of near work compared to those who use their eyes less for such activities. Longer durations of education are also associated with higher myopia prevalence. This may be related to hyperopic defocus caused by accommodation lag during near work, binocular vision dysfunction, and incomplete relaxation of accommodation.
Outdoor Activities and Light Exposure
Epidemiological studies indicate that outdoor activities and adequate illumination can reduce the incidence of myopia. Increased dopamine release in the retina under light exposure may inhibit axial elongation. Factors such as the wavelength and intensity of natural outdoor light, pupil constriction in outdoor settings, increased depth of field, and improved image quality may contribute to delaying the onset of myopia.
Peripheral Refraction and Image Quality
Due to the shape of the eyeball, when on-axis light is focused on the macula, off-axis light may form images behind the peripheral retina (hyperopic defocus). This can lead to accelerated local growth of the peripheral eyeball, resulting in axial elongation. Additionally, the presence of ocular wavefront aberrations and reduced image quality on the retina may also contribute to the development and progression of myopia.
Nutrition and Diet
Dietary patterns high in sugar and fat may contribute to the progression of myopia. Studies have reported associations between myopia progression and factors such as glucagon and insulin-like growth factor (IGF), as well as blood concentrations of saturated fatty acids, cholesterol, vitamin D, zinc, and other trace elements, which may correlate with axial elongation.
Classification
Classification by Degree of Myopia
Myopia can be divided into:
- Mild Myopia: -0.50D ≤ Spherical Power < -3.00D
- Moderate Myopia: -3.00D ≤ Spherical Power < -6.00D
- High Myopia: Spherical Power ≥ -6.00D
Classification by Refractive Components
Myopia can be divided into:
- Refractive Myopia: Can be subdivided by etiology into two types:
- Curvature Myopia: Characterized by an excessively small radius of curvature on the corneal front surface or the lens surface.
- Refractive Index Myopia: Characterized by an abnormally high refractive index (i.e., increased refractive power) of the cornea or lens, leading to the focal point of parallel light beams falling in front of the retina after refraction.
- Axial Myopia: Defined by an excessive axial length of the eyeball beyond the normal range. In this subtype, the curvature and refractive indices of the cornea and lens remain within normal limits. Axial myopia is typically observed in pathological myopia and most cases of simple myopia.
Classification by Disease Progression and Pathological Characteristics
Myopia can be divided into:
- Simple Myopia: Characterized by a myopic degree generally within -6.00D, with minimal or no pathological changes observed in the fundus.
- Pathological Myopia: Characterized by a myopic degree exceeding -6.00D with continuous progression. In these cases, axial elongation is excessive (generally >26.5mm) and frequently accompanied by fundus lesions that impair vision.
Clinical Manifestations
Myopia primarily presents with blurred distance vision while near vision remains clear. Early in the onset of myopia, distance vision may fluctuate, and patients often squint to see distant objects. As accommodation is either unnecessary or minimally required for near vision, convergence of both eyes correspondingly decreases, potentially leading to exophoria or exotropia. During childhood and adolescence, the degree of simple myopia often progresses but typically remains within -6.00D, with minimal or no pathological changes in the fundus. Best-corrected visual acuity is generally normal or near-normal at this stage.
High myopia frequently progresses into pathological myopia, which is a major cause of irreversible vision loss. It is characterized by the continuous increase in myopic degree, often with best-corrected visual acuity falling below normal levels. Patients may experience symptoms such as poor night vision, floaters, visual disturbances, and photopsia. Excessive axial elongation (commonly >26.5mm) and pathological changes in the fundus may occur, including tigroid fundus, lacquer cracks, Fuchs' spots, lattice degeneration of the peripheral retina, subretinal neovascularization, and posterior staphyloma. Compared to individuals with normal vision, pathological myopia patients are at a younger age of onset for vitreous liquefaction, opacities, and posterior vitreous detachment. They also face significantly increased risks of retinal detachment, macular hemorrhages, glaucoma, and cataracts.
Diagnosis
Myopia can be diagnosed when refractive error results indicate a degree of ≥-0.50D, combined with the described clinical manifestations. For first-time patients or children, cycloplegic refraction (commonly known as "dilated refraction") is recommended to exclude the effects of overaccommodation. To further evaluate the type, progression, and complications of myopia, additional tests may include intraocular pressure measurement, morphological assessments (e.g., axial length, corneal curvature, optical coherence tomography), visual function evaluations (including accommodation, vergence, and visual fields), and fundus photography. For high myopia, emphasis should be placed on monitoring the fundus, intraocular pressure, axial length, and visual fields, with timely intervention based on disease progression.
Management
Since myopia is a progressive condition, effective monitoring is necessary, which includes assessing risk factors for the onset and progression of myopia, as well as tracking eye health. For children and adolescents at pre-myopic stages (i.e., those not yet myopic but exhibiting risk factors or accelerated eye growth indicative of high myopia risk), preventive measures should be taken to promote good eye habits and conduct regular evaluations. Studies suggest that age-matched hyperopic reserve is a key predictor for myopia onset. A reduced hyperopic reserve indicates an increased likelihood of developing myopia.
For individuals who are already myopic, management involves both lifestyle intervention and clinical treatment. Current clinical interventions to slow myopia progression include functionally designed eyeglasses (e.g., defocus-designed lenses), orthokeratology lenses, multifocal soft contact lenses, and low-dose atropine eye drops, all of which have been implemented in clinical practice.
Correction
The globally recognized methods for refractive correction include spectacles, contact lenses, and refractive surgeries. The optical principle of these methods is to adjust the eye's refractive power using various lenses or by altering the refractive properties of the eye, allowing light rays from external objects to focus clearly on the retina.
Spectacles
Spectacles consist of a frame and lenses, making them the most common refractive correction device. They also serve as protective equipment for the eyes.
Types of Spectacles
Single Vision Lenses
These lenses have a uniform refractive power across the entire surface. Typically crescent-shaped, single vision lenses have a convex front surface and a concave back surface. Concave lenses are used for myopia correction. Single vision lenses are relatively inexpensive, easy to manufacture, and simple to use.
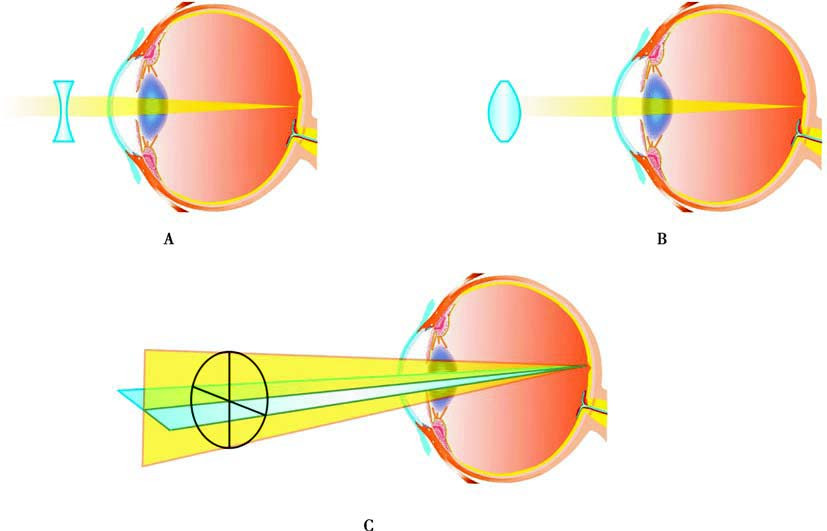
Figure 2 Principles of correcting refractive errors with spectacles
A. Concave lenses for correcting myopia
B. Convex lenses for correcting hyperopia
C. Spherocylindrical lenses (toric lenses) for correcting astigmatism
Functional Spectacles
These lenses are specially designed with multifocal or defocus features, where different refractive powers are distributed across a single lens or central and peripheral refractive powers follow different designs. Besides correcting myopia, these lenses also help slow the progression of the condition.
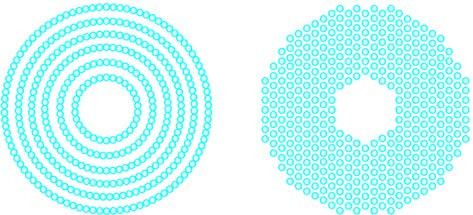
Figure 3 Design of functional spectacle frames
Prescription of Spectacles
The prescription for spectacles is generally determined based on subjective refraction results, with adjustments made through trial lens fitting in a trial frame. The final prescription is written according to specific conventions, indicating the eye being corrected and listing the prescription for the right eye first, followed by the left eye. The right and left eyes are denoted by "R" (right eye) and "L" (left eye), or by the Latin abbreviations "OD" (oculus dexter, meaning right eye), "OS" (oculus sinister, meaning left eye), and "OU" (oculus uterque, meaning both eyes). Prescriptions typically include distance correction first, followed by near correction.
For patients with both myopia and astigmatism, spherical-cylindrical correction may be used. The spherical power (denoted as DS, diopter of spherical power) is recorded to two decimal places. Cylindrical power (denoted as DC, diopter of cylindrical power) is also recorded to two decimal places and accompanied by the axis of the cylinder, indicating the meridian with the least refractive power. Prisms, when required, are specified by their power in prism diopters (denoted as Δ), along with the direction of the prism base. Prism base directions include up, down, inward (nasal), or outward (temporal), recorded as Base Up (BU), Base Down (BD), Base In (BI), and Base Out (BO), respectively.
When a prescription includes components for spheres, cylinders, or prisms, their combination may be expressed using a forward slash (/). For example:
OD: -6.50DS / -1.75DC × 170 / 3Δ BD
OS: -6.50DS / -1.00DC × 180
This prescription indicates that for the right eye (OD): -6.50 diopters of spherical power is combined with -1.75 diopters of cylindrical power at an axis of 170°, and the lens includes 3 prism diopters with the base oriented downward. For the left eye (OS): -6.50 diopters of spherical power is combined with -1.00 diopters of cylindrical power at an axis of 180°.
During the fitting of spectacles, measurements such as binocular pupillary distance (PD) and vertical pupil height need to be obtained. The distance between the optical centers of the lenses must match the patient's PD, ensuring that the optical center of the lens aligns with the center of the patient’s pupils; otherwise, unwanted prism effects may occur. For functional spectacles or aspheric single-vision lenses, monocular pupil height may also need to be assessed. In cases of myopia combined with strabismus, the method of "decentration," where the optical center of the lens is deliberately offset from the pupil center, may be applied to correct the strabismus.
For patients with high refractive error or anisometropia, image magnification must be considered when using spectacles. For negative lenses (used to correct myopia), the higher the lens power, the smaller the resultant image. Conversely, for positive lenses (used to correct hyperopia), the higher the lens power, the larger the resultant image.
Cylindrical Prescription Conversion
Cylindrical prescriptions may occasionally require conversion, wherein the mathematical form of the prescription is changed without altering the corrective effect. The conversion follows three principles collectively known as the "sum-sign-axis" rule:
The algebraic sum of the original spherical and cylindrical powers becomes the new spherical power.
The sign of the cylindrical power is reversed (positive changes to negative, or negative changes to positive).
The axis of the cylinder is rotated to the perpendicular meridian (add 90° if the original axis is ≤90°, or subtract 90° if the original axis is >90°).
In clinical settings, the negative cylinder form is commonly used. For example, a prescription of -5.00DS / +1.50DC × 75 is converted to -3.50DS / -1.50DC × 165.
Contact Lenses
Contact lenses are worn directly on the anterior corneal surface and are categorized by material into soft contact lenses (SCLs) and rigid contact lenses (RCLs). Key parameters for contact lens fitting include diameter, base curve (the radius of curvature of the optical zone on the back surface of the lens), and refractive power. Specially designed contact lenses hold unique clinical value in conditions like corneal pathology, color blindness, trauma, postoperative care, ocular drug delivery, and clinical interventions for myopia. For example, colored contact lenses may assist patients with color blindness, artificial pupil lenses may benefit those with iris defects, bandage lenses may aid postoperative care, and drug-releasing lenses may facilitate sustained drug delivery to the eye. Multifocal soft lenses and orthokeratology lenses are the primary modalities for myopia management.
Soft Contact Lenses
Soft contact lenses are made from hydrophilic polymer materials. For conventional soft lenses, oxygen permeability depends on the material's water content and lens thickness. The use of silicone hydrogel materials significantly improves oxygen permeability while reducing dependence on water content. Soft lenses typically have a diameter of 13.5–14.5mm and a base curve radius of 8.4–8.8mm.
Soft lenses are characterized by their flexibility and initial comfort. Based on replacement schedules, they can be classified into:
- Conventional Lenses: Worn continuously for 7–30 days with extended replacement cycles.
- Planned Replacement Lenses: Replaced every 2 weeks to 3 months.
- Disposable Lenses: Designed for single use or disposed of after 1–2 weeks of wear.
Modern soft lenses generally adopt planned replacement or disposable formats. Proper fitting, lens maintenance, and shorter replacement cycles are essential to reduce the risk of contact lens-related complications.
Rigid Contact Lenses
Modern rigid contact lenses, known as rigid gas-permeable contact lenses (RGPCLs), are characterized by their high oxygen permeability and rigid material. RGP lenses are smaller in diameter compared to soft lenses, typically ranging from 9.2 to 9.6 mm. Their back surface design aligns with the curvature of the anterior corneal surface. The fitting of rigid lenses requires advanced technical expertise, and users often need a period of adaptation. RGP lenses offer high oxygen permeability, strong resistance to protein deposits, and the ability to maintain their shape on the cornea, resulting in superior optical image quality. Additionally, the tear film layer between the lens and the cornea acts as a "tear lens," providing refractive power that can correct corneal astigmatism (both regular and irregular). These features make RGP lenses suitable for correcting vision in specific conditions, such as keratoconus, irregular corneas caused by trauma, and post-corneal refractive surgery. RGP lenses also have the advantage of customizable parameters to fit unique corneal shapes.
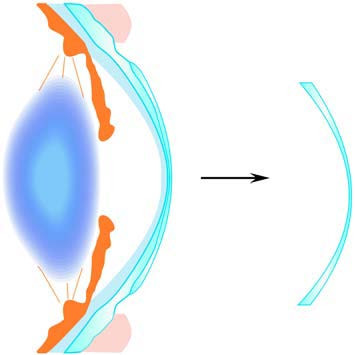
Figure 4 Formation of the tear lens by rigid lenses, tear film, and cornea
Orthokeratology (Ortho-K) is a specially designed type of rigid contact lens that employs reverse geometry to temporarily reshape the corneal curvature. Through mechanical pressure, lens movement-induced massage, and tear film hydraulics, these lenses reduce myopia, improving unaided visual acuity. Orthokeratology is typically used for individuals with myopia up to -6.00D. The effects of Ortho-K are reversible, with refractive errors returning to their original levels after discontinuation of use. Clinical studies have also indicated that long-term use of ortho-k lenses may slow the progression of myopia, making them an important clinical intervention method in myopia management.
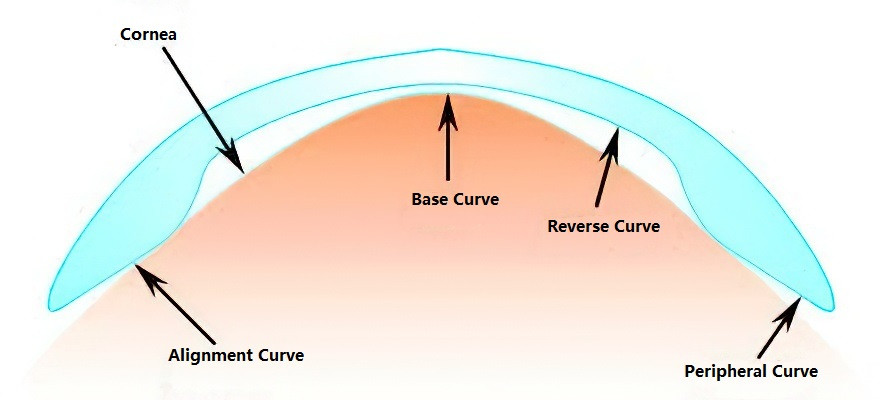
Figure 5 Design principles of orthokeratology lenses
The fitting of orthokeratology lenses is most commonly performed for children and adolescents with myopia. Medical institutions providing this service are expected to have advanced equipment, skilled healthcare professionals, and to carefully select appropriate candidates. They are required to use certified lenses, adhere to standardized fitting procedures, and provide thorough follow-up care.
Pharmacological Strategies
Recent research on low-concentration atropine has demonstrated its utility in the control of myopia progression. Currently, 0.01% atropine eye drops have been approved for clinical use. Since they are prescription medications, they must be used under the guidance of an ophthalmologist.
Refractive Surgery
Refractive surgery alters the refractive status of the eye through surgical means. Based on the surgical location, refractive surgeries are categorized into corneal refractive surgery, intraocular refractive surgery, and scleral refractive surgery. Modern refractive surgeries can involve combined methods, multi-technology designs, or stepwise implementation. While most refractive errors can be satisfactorily corrected by non-surgical methods such as spectacles or contact lenses, patients opting for refractive surgery generally have high expectations. Institutions performing such surgeries are required to maintain advanced surgical equipment and well-trained specialists. Surgeons are responsible for ensuring both the safety and efficacy of the procedure, strictly assessing candidates, and conducting thorough preoperative consultations to explain the expected outcomes and risks, thereby minimizing complications.
Corneal Refractive Surgery
Corneal refractive surgery refers to procedures that reshape the anterior surface of the cornea to correct refractive errors.
Non-Laser Corneal Refractive Surgery
Non-laser corneal refractive surgeries include radial keratotomy (RK), intrastromal corneal ring segments (ICRS), astigmatic keratotomy (AK), and corneal collagen cross-linking (CXL). CXL plays an essential role in the treatment of keratoconus and related conditions. During this procedure, riboflavin (vitamin B2) is used as a photosensitizing agent, and ultraviolet light generates reactive oxygen species, which facilitate covalent bonding between adjacent collagen fibers. This increases the biomechanical strength of the cornea, reducing corneal deformation and irregular astigmatism.
Laser Corneal Refractive Surgery
Laser corneal refractive surgery employs laser ablation of the corneal stroma to reshape the cornea, altering its radius of curvature to correct refractive errors. Patients are typically required to be at least 18 years old with stable refraction for two or more years. Laser corneal surgeries are broadly divided into two categories:
- Surface Ablation: This involves the removal of the corneal epithelium to expose Bowman’s layer, followed by laser ablation. Photorefractive keratectomy (PRK) is a representative procedure of this type.
- Lamellar (Stromal) Ablation: This involves creating a corneal flap, lifting it, and performing laser ablation on the stroma. Examples include femto-LASIK (femtosecond-assisted laser in situ keratomileusis) and SMILE (small incision lenticule extraction).
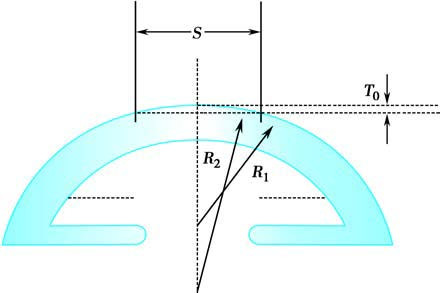
Figure 6 Principles of PRK in treating myopia
S: Ablation width; T0: Ablation depth; R1: Radius of curvature of the anterior corneal surface before ablation; R2: Change to a flatter anterior corneal surface due to increased radius of curvature after ablation.
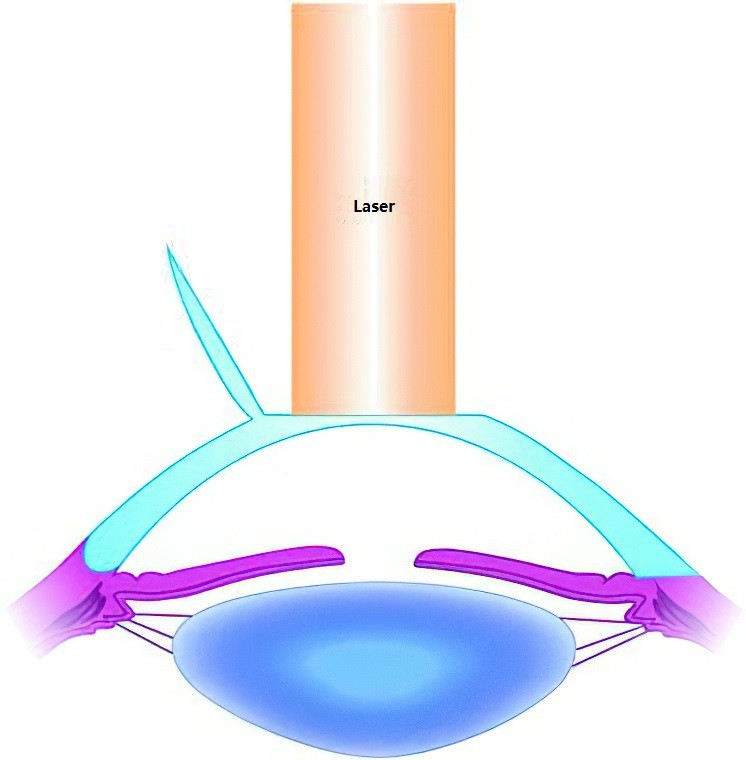
Figure 7 Principles of LASIK in treating myopia
Intraocular Refractive Surgery
Intraocular refractive surgery modifies refractive status by operating on the crystalline lens or the anterior and posterior chambers. This type of surgery accommodates a wider range of refractive errors compared to corneal refractive surgery, typically ranging from +10.00D to -20.00D. Procedures are categorized into two types based on whether the natural lens is preserved.
Refractive Lens Exchange (RLE)
This method involves removing the crystalline lens, either transparent or cataractous, and implanting an intraocular lens in the capsular bag within the posterior chamber. This surgery is an option for patients unsuitable for corneal refractive surgery, such as those with high myopia or hyperopia.
Phakic Intraocular Lens Implantation
This method is suitable for patients with stable refractive status who are not candidates for or decline spectacles, contact lenses, or corneal refractive surgery. The lens is implanted in either the anterior or posterior chamber. Posterior chamber phakic intraocular lenses typically feature a single-piece posterior arch design that conforms to the anterior surface of the natural lens while maintaining adequate spacing between the implant and the natural lens.
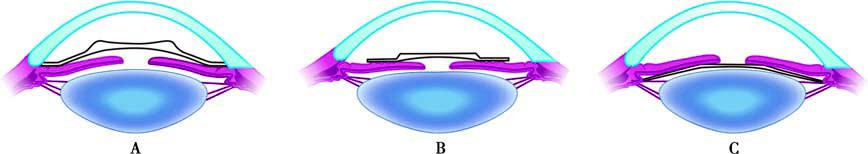
Figure 8 Types of phakic intraocular lenses used in phakic intraocular lens implantation
A. Anterior chamber angle-supported intraocular lenses;
B. Iris-claw intraocular lenses;
C. Posterior chamber intraocular lenses.
Scleral Refractive Surgery
Posterior scleral reinforcement (PSR), also known as posterior scleral buckling or posterior scleral strengthening surgery, involves using biological or synthetic materials, either autologous or allogenic, to reinforce the posterior sclera of the eyeball. The purpose is to slow the progression of myopia. Progressive myopia patients with refractive errors of -8.00D to -10.00D or greater, and a yearly myopic progression of 0.50D to 2.00D or more, may be considered for this procedure.