Age-related macular degeneration (AMD) primarily affects individuals over the age of 50 and typically manifests in one or both eyes, either sequentially or simultaneously, with progressive loss of visual acuity. AMD is the leading cause of irreversible vision loss in the elderly population, and its incidence increases with advancing age.
Etiology and Pathophysiology
The exact cause of AMD remains unknown, but potential contributing factors include genetic predisposition, chronic light-induced damage to the macula, metabolic influences, and nutritional deficiencies. The primary pathophysiological changes involve degenerative processes in the outer retina, retinal pigment epithelium (RPE), Bruch's membrane, and the choriocapillaris. Extracellular deposits in the macular region are characteristic findings. Early pathological features include basal laminar deposits and basal linear deposits, whereas advanced stages may present with atrophy of the retina or choroid or the formation of new blood vessels.
Clinical Features
AMD has two distinct clinical types:
Dry AMD
Also referred to as atrophic or non-neovascular AMD, this type progresses slowly, with bilateral and gradual decline in visual acuity, often accompanied by distorted vision. The disease is characterized by progressive degeneration and atrophy of the outer retina, RPE layer, Bruch's membrane, and the choriocapillaris in the posterior pole. Key features include drusen in the macular region, pigmentary disturbances, and geographic atrophy. In the early stages, the posterior pole shows drusen of varying sizes and a yellowish-white, round appearance. Hard drusen are small with well-defined edges, whereas soft drusen are larger with indistinct margins that may expand and merge. Soft drusen are considered risk factors for RPE atrophy and exudative AMD. RPE degeneration and atrophy may further present with pigmentary disturbances, depigmentation, or geographic atrophy. Atrophy of the choriocapillaris may expose underlying large or medium choroidal vessels.
Wet AMD
Also known as exudative or neovascular AMD, this type arises from degenerative changes in Bruch's membrane, which may induce choroidal neovascularization (CNV). These neovascular vessels may grow beneath the RPE or subretinal layer, leading to exudative or hemorrhagic detachment, and eventually to fibrotic scar formation. Clinically, this type is characterized by a sudden decline in visual acuity, distorted vision, or central scotomas. Fundus examination may reveal subretinal or sub-RPE dark red to black hemorrhages in the posterior pole. The lesion may appear elevated, vary in size, and sometimes extend beyond the vascular arcades. Yellowish-white hard exudates and drusen can be observed within or around the lesion. In cases of extensive bleeding, hemorrhage may extend into the vitreous cavity, leading to vitreous hemorrhage. In the late stage, submacular hemorrhage undergoes fibrosis, forming a disciform scar and causing central vision loss.
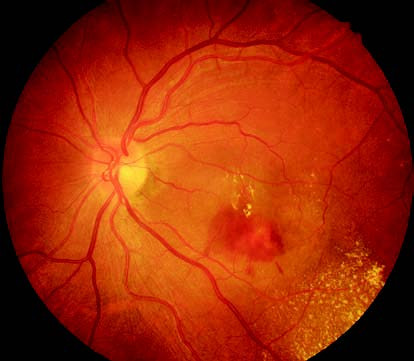
Figure 1 Fundus photograph of the left eye with wet age-related macular degeneration
Subretinal dark red hemorrhage is observed in the macular region, with the lesion appearing elevated. Yellowish-white lipid exudates are visible around the lesion.
Fluorescein fundus angiography (FFA) can not only detect CNV but also distinguish its types (classic and occult). Classic CNV appears early during angiography as lace-like or ball-like vessels with well-defined boundaries, followed by fluorescein leakage and blurred margins. In contrast, occult CNV demonstrates fluorescence leakage in the mid-to-late stages of angiography, appearing as poorly defined areas of intense fluorescence. Indocyanine green angiography (ICGA) provides enhanced visualization of CNV, allowing further classification of occult CNV into focal, patchy, or mixed types based on angiographic findings.
Special Subtypes of Wet AMD
Polypoidal Choroidal Vasculopathy (PCV)
The pathogenesis of PCV remains unclear, and it is debated whether it represents a subtype of AMD or a distinct disease entity. In typical cases, fundus examination reveals orange-red, nodular lesions in the posterior pole, often accompanied by hemorrhage, exudation, and pigment epithelial detachment. Large pigment epithelial detachments in PCV may be associated with extensive subretinal hemorrhage, leading to poor prognosis. ICGA is considered the "gold standard" for PCV diagnosis. The presence of one or multiple polyp-like lesions originating from the choroidal circulation, with or without an associated branch vascular network (BVN), confirms the diagnosis. OCT may reveal features such as "finger-like projections" or a "double-layer sign," further aiding in its diagnosis.
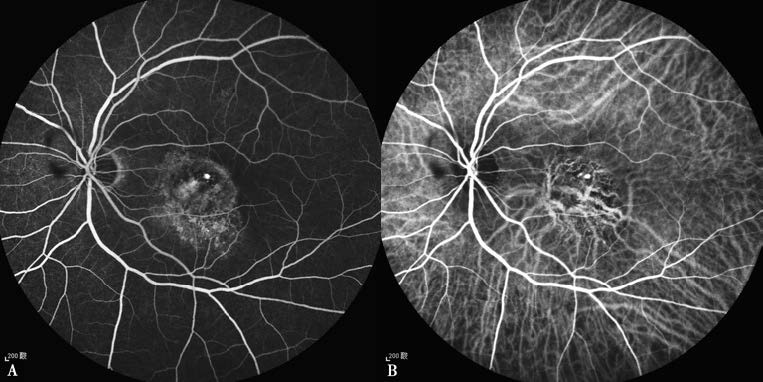
Figure 2 Fluorescein angiography (FFA) and indocyanine green angiography (ICGA) of polypoidal choroidal vasculopathy in the left eye
A. FFA reveals nodular hyperfluorescence in the macular area accompanied by surrounding fluorescein leakage.
B. ICGA shows nodular polyp-like hyperfluorescent lesions with enlarged and dilated choroidal capillaries and a branching vascular network.
Retinal Angiomatous Proliferation (RAP)
RAP is currently regarded by most researchers as a unique subtype of AMD. Unlike typical AMD, its pathological hallmark is the formation of new blood vessels originating from the retinal neuroepithelium's capillaries, accompanied by dilated retinal vessels as well as pre-retinal, intraretinal, and subretinal hemorrhages and exudates. This angiomatous proliferation extends to the deep retina and subretinal space, forming retinal-choroidal anastomoses. The prognosis for RAP is generally poor.
Updated Concepts
Considering that the neovascular origin in the special subtype of AMD, retinal angiomatous proliferation (RAP), arises from the retina, the term "CNV" (choroidal neovascularization) does not fully capture all types of neovascular growth associated with AMD. In 2020, the International AMD Nomenclature Consensus Group proposed replacing "CNV" with the term macular neovascularization (MNV). MNV refers to neovascular growth extending into the macular retina, subretinal space, or the sub-RPE space. Based on the origin and location of MNV on OCT images, MNV is classified into Types 1, 2, and 3. The new terminology corresponds to previously recognized disease definitions to some extent.
Type 1 MNV originates from the choriocapillaris and grows into the sub-RPE space. On OCT, it is visible beneath the RPE layer and was previously referred to as occult CNV. An important subtype of Type 1 MNV is polypoidal choroidal vasculopathy (PCV), which shares OCT features with Type 1 MNV but also demonstrates aneurysmal dilation or a branching vascular network on ICGA. This subtype is termed aneurysmal Type 1 MNV.
Type 2 MNV arises from the choriocapillaris, penetrates the RPE, and grows into the subretinal space. On OCT, it is located above the RPE layer and was previously referred to as classic CNV. Type 2 MNV may coexist with other types of MNV.
Type 3 MNV, previously known as RAP, refers to abnormal vessels that do not originate from the choroid but instead are thought to arise from the retinal deep capillary plexus, extending into the outer retina. On OCT, these neovascular vessels are seen proliferating towards the RPE layer and, in some cases, penetrating through the RPE.
Diagnosis and Differential Diagnosis
A combination of a detailed medical history, routine ophthalmic examinations, and multimodal imaging—including FFA, ICGA, OCT, and OCTA—is typically sufficient to confirm the diagnosis. Due to their advantages of being non-invasive, convenient, safe, and repeatable, OCT and OCTA have become essential tools for clinically diagnosing and monitoring AMD. In cases of AMD with substantial subretinal hemorrhage, differentiation from choroidal melanoma may be necessary.
Treatment
Dry AMD
Patients with dry AMD may benefit from supplementation with antioxidants (Vitamin C, Vitamin E), minerals (zinc and copper), lutein, and zeaxanthin. Laser photocoagulation or subthreshold micropulse laser treatment may promote absorption of soft drusen.
Wet AMD/MNV
For MNV, intravitreal injection of anti-VEGF agents is the first-line treatment. These therapies act by inhibiting VEGF and have demonstrated significant efficacy. However, frequent recurrence necessitates multiple injections. Photodynamic therapy (PDT), which induces a photochemical reaction using specific photosensitizers, may cause cytotoxic damage to vascular endothelial cells and destroy neovascular tissue, though it does not prevent severe vision loss in the affected eye. For MNV lesions located outside the foveal center, both anti-VEGF treatment and retinal laser photocoagulation are viable options.
Surgical Options
Macular surgery options include the removal of subretinal hemorrhage, excision of CNV, or macular translocation surgery, though therapeutic outcomes remain uncertain and require further evaluation.
PCV Treatment
Currently, the most effective treatment for PCV is either stand-alone anti-VEGF therapy or a combination of anti-VEGF therapy with photodynamic therapy. For polypoidal lesions located outside the foveal center, laser photocoagulation may be considered.
Advanced AMD with Severe Vision Loss
For patients with severe vision impairment, low-vision rehabilitation may be offered.