Primary glaucoma refers to a category of glaucoma for which the underlying mechanisms are not yet fully understood. Based on the status of the anterior chamber angle during intraocular pressure elevation—whether it is closed or open—it can be further classified into primary angle-closure glaucoma and primary open-angle glaucoma.
Primary Angle-Closure Glaucoma (PACG)
Primary angle-closure glaucoma is a type of glaucoma caused by peripheral iris obstruction of the trabecular meshwork or permanent adhesion to the trabecular meshwork, leading to impaired aqueous outflow, elevated intraocular pressure, and resulting damage to the optic nerve and visual field. Affected eyes typically exhibit anatomical characteristics such as shallow anterior chambers and narrow anterior chamber angles. Based on whether the rise in intraocular pressure occurs suddenly or gradually, PACG can be further divided into acute and chronic forms.
Risk Factors for Primary Angle-Closure Glaucoma
The pathogenesis of PACG is highly complex and involves genetic, physiological, and environmental factors. Similar to primary open-angle glaucoma, the prevalence of PACG increases with age and is higher in females than in males. With aging, forward displacement of the lens, increased pupillary block, and narrowing of the anterior chamber angle can occur. For acute PACG, pupillary block is the most significant contributing factor. Additionally, a family history of angle-closure glaucoma and hyperopia are risk factors for this condition. Certain genetic variants associated with angle-closure glaucoma have been identified, including genes like COL11A1 (collagen type XI alpha 1 chain) and PLEKHA7 (pleckstrin homology domain containing A7).
Patients over the age of 40 presenting with a combination of small corneal diameters, shallow anterior chambers, forward lens displacement, and anteriorly located ciliary bodies should be monitored closely for changes in intraocular pressure and anterior chamber angles.
Mechanisms of Anterior Chamber Angle Closure in PACG
Angle closure in PACG can occur via pupillary block, non-pupillary block mechanisms, or a combination of multiple mechanisms. Subclassifying these mechanisms provides valuable guidance for clinical management.
Pupillary Block Mechanism
This occurs when the iris is in close contact with the anterior surface of the lens, increasing resistance to aqueous humor flow through the pupil and preventing its entry into the anterior chamber. This results in increased pressure in the posterior chamber, causing the peripheral iris to bulge forward, narrowing or closing the anterior chamber angle. This mechanism is clinically associated with subacute or acute attacks. Following peripheral iridectomy, aqueous humor in the posterior chamber bypasses the pupil through the iridectomy opening, equalizing pressure between the posterior and anterior chambers. This flattens the peripheral iris and widens or reopens the anterior chamber angle. Mapstone and Kondo proposed a mathematical formula for measuring relative pupillary block force through mechanical analysis.
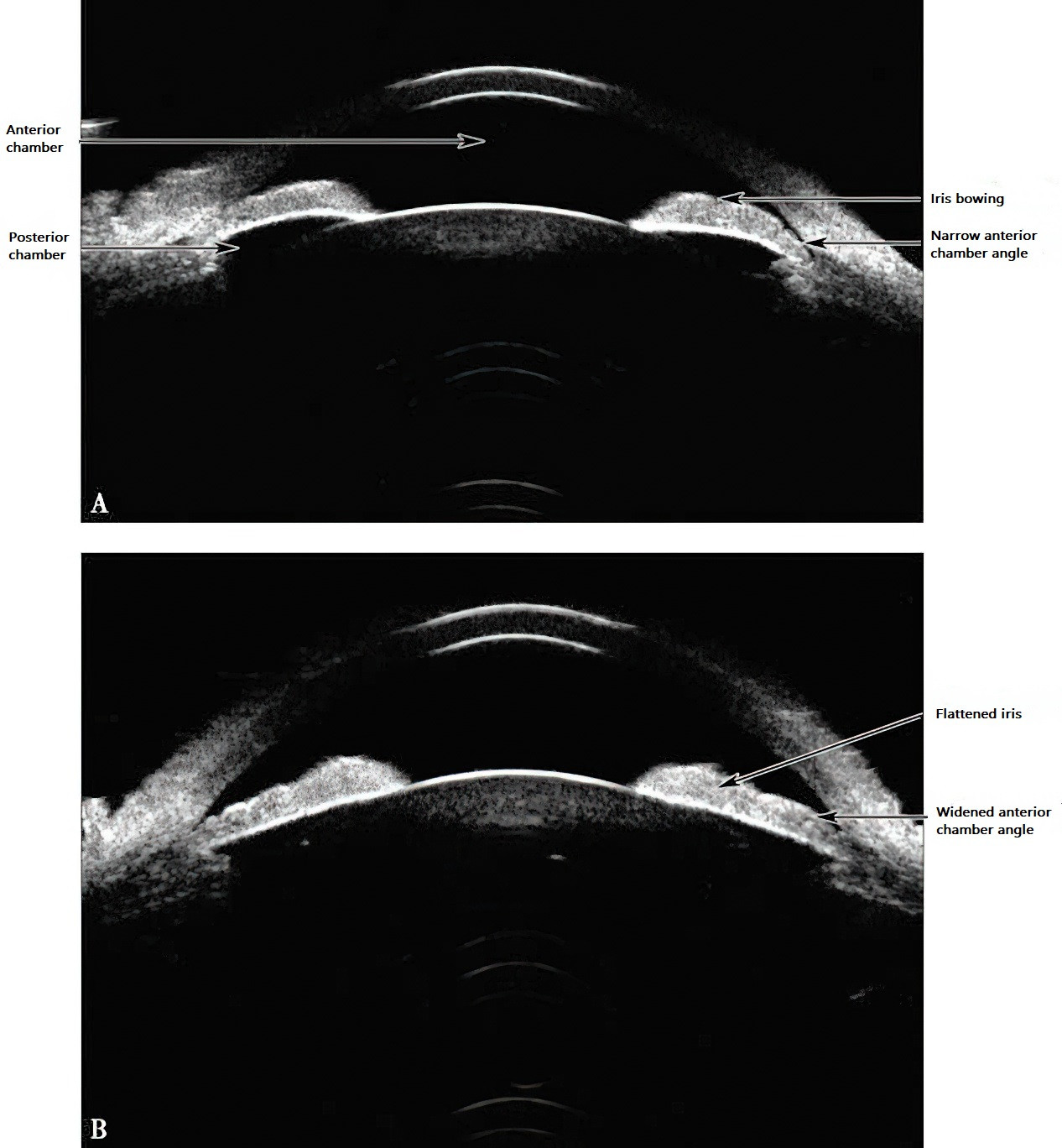
Figure 1 Mechanism of pupillary block in primary angle-closure glaucoma
A. Increased pupillary block force restricts the flow of aqueous humor from the posterior chamber to the anterior chamber through the pupil, raising the pressure in the posterior chamber. This pressure causes the iris to bow forward, leading to further narrowing or complete closure of the anterior chamber angle.
B. After peripheral iridectomy, aqueous humor from the posterior chamber flows directly into the anterior chamber through the iridectomy site, balancing the pressure between the anterior and posterior chambers. The peripheral iris flattens, and the anterior chamber angle reopens or widens.
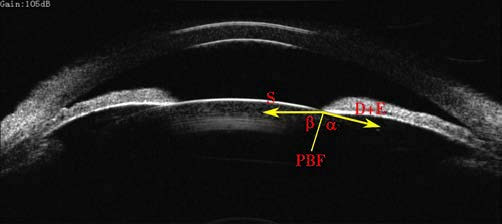
Figure 2 Schematic representation of measuring relative pupillary block force (PBF)
PBF = (D+E)cosα + S cosβ
Where:
PBF represents the pupillary block force.
D refers to the force of the dilator muscle.
E represents the tension of the iris.
S refers to the force of the sphincter muscle.
Angle α is the angle between the vector direction of (D+E) and the line connecting the pupil margin to the center of curvature on the front surface of the lens.
Angle β is the angle between the vector direction of S and the aforementioned line.
Non-Pupillary Block Mechanisms
These can be further divided into mechanisms involving peripheral iris thickening or forward positioning of the ciliary body. In cases involving peripheral iris thickening, the thickened iris root forms a trapezoidal structure that narrows the anterior chamber angle, leading to its obstruction. Some researchers refer to this condition as "iris plateau syndrome." Forward positioning of the ciliary body causes the ciliary body to push the peripheral iris anteriorly, thereby narrowing or closing the anterior chamber angle.
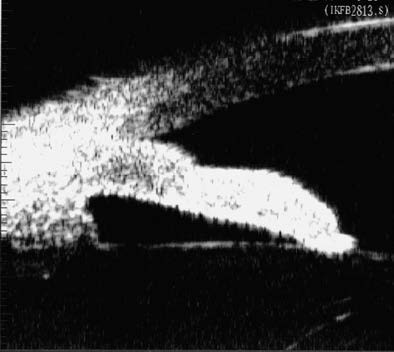
Figure 3 Peripheral iris thickening (iris folding)
When the pupil is moderately dilated, the thickened peripheral iris accumulates and bulges at the anterior chamber angle, resulting in further narrowing or closure of the angle.
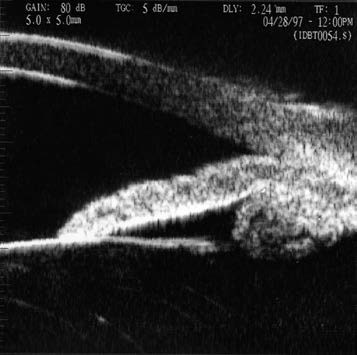
Figure 4 Anterior positioning of the ciliary body
Ultrasound biomicroscopy (UBM) images demonstrate anterior displacement of the ciliary body, pushing the peripheral iris towards the anterior chamber angle, causing angle narrowing or closure.
Mechanisms Involving Multiple Causes
This includes combinations such as pupillary block with peripheral iris thickening, pupillary block with forward ciliary body displacement, or pupillary block with both peripheral iris thickening and forward ciliary body displacement.
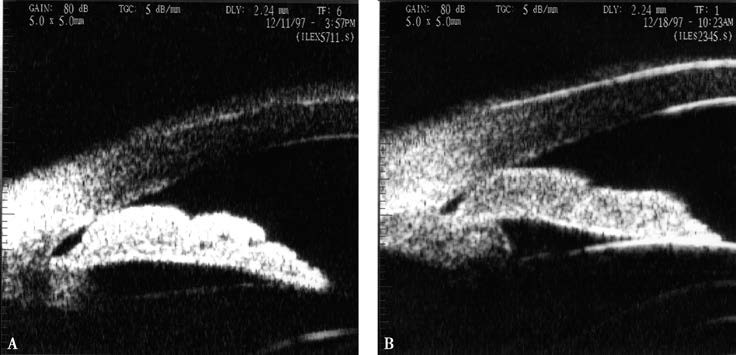
Figure 5 Coexistence of multiple mechanisms
A. Pupillary block combined with accumulation of thickened peripheral iris.
B. After peripheral iridectomy, the iris flattens, but the thickened peripheral iris accumulates at the anterior chamber angle, narrowing the angle.
Provocative Testing for Primary Angle-Closure Glaucoma
Dark Room Provocation Test
This test is designed for screening PACG. Participants sit quietly in a dark room for one to two hours, and an intraocular pressure increase of ≥8 mmHg is interpreted as positive. It is generally believed that the intraocular pressure elevation in the dark is caused by pupil dilation, peripheral iris thickening, and subsequent narrowing or obstruction of the anterior chamber angle.
Dark Room Prone Provocation Test
In a dark room, intraocular pressures are measured in both eyes before the test. The patient is then instructed to lie prone on an examination bed while wearing an eye mask. The patient's back remains level, and ocular compression is avoided. After 1.5 hours, intraocular pressure is measured again, with an increase of ≥8 mmHg regarded as positive. This test is based on the mechanism of pupil dilation and anterior chamber angle obstruction in the dark, combined with forward displacement of the lens-iris diaphragm due to the prone position, which narrows the anterior chamber angle and causes an increase in intraocular pressure. Clinical experience indicates that the sensitivity of this test can be low, possibly due to the iris sphincter muscle becoming abnormally sensitive to light after prolonged time in darkness. Even the dim light of the slit-lamp microscope used during subsequent gonioscopy can induce pupil constriction, altering the configuration of the anterior chamber angle and reducing test sensitivity. Research has shown that the iris sphincter muscle exhibits the lowest light sensitivity three minutes after entering a dark room. Therefore, combining three-minute dark room gonioscopy for anterior chamber angle evaluation with intraocular pressure assessment following the 1.5-hour prone test can significantly improve the test’s sensitivity and specificity.
Acute Angle-Closure Glaucoma
Acute angle-closure glaucoma is an eye disorder characterized by a sudden closure of the anterior chamber angle, leading to a rapid elevation in intraocular pressure along with associated symptoms and pathological changes in the anterior segment tissues. This condition is more common in individuals over 50 years of age, with a higher incidence in females than males, at a ratio of approximately 1:2. Patients often present with hyperopia, and the condition may affect both eyes either simultaneously or sequentially. Triggers such as emotional stress, prolonged exposure to dark environments, or the use of local or systemic anticholinergic medications can induce pupillary dilation and peripheral iris relaxation, leading to the onset of this condition. Other common predisposing factors include prolonged reading, fatigue, and pain.
Etiology
The underlying causes of acute angle-closure glaucoma are not yet fully understood. Anatomical abnormalities of the eye are widely recognized as major risk factors. These inherited anatomical variations include a shorter axial length, smaller corneal diameter, shallow anterior chamber, narrow angle, and thicker lens. With aging, the lens increases in thickness, the anterior chamber becomes shallower, and pupillary block worsens, resulting in a higher likelihood of angle-closure glaucoma. Once the peripheral iris comes into contact with the trabecular meshwork, the angle becomes closed, leading to a rapid elevation in intraocular pressure and triggering an acute attack.
Clinical Presentation and Stages
Typical acute angle-closure glaucoma progresses through several clinical stages, each with distinct features and treatment principles:
Prodromal Stage
Acute angle-closure glaucoma is a bilateral condition. If one eye experiences an acute attack and is diagnosed, the contralateral eye, even in the absence of symptoms, can be diagnosed as being at the prodromal stage of angle-closure glaucoma. Additionally, some patients with angle-closure glaucoma may not exhibit subjective symptoms before an acute attack but may show features like a shallow anterior chamber, iris convexity, and narrow angles, particularly after provocation tests like the dark room test that reveal a significant rise in intraocular pressure. These cases can also be classified as being in the prodromal stage.
Pre-Attack Stage
This stage involves transient or recurrent minor attacks. Symptoms typically appear in the evening and may include sudden blurring of vision ("foggy vision"), colored halos around lights, mild headache near the affected eye, or a sense of pressure at the nasal bridge on the same side. These symptoms are short-lived and often resolve spontaneously with rest. If examined during an episode, findings may include elevated intraocular pressure, often exceeding 40 mmHg, mild or no hyperemia, slight corneal epithelial edema with a mild hazy appearance, and an extremely shallow anterior chamber. Aqueous humor typically remains clear, with extensive angle closure, a slightly dilated pupil, and a sluggish light reflex. After resolution of minor attacks, the eye generally does not exhibit permanent damage, except for the characteristic shallow anterior chamber.
Acute Attack Stage
Symptoms include severe headache, eye pain, photophobia, tearing, and marked vision reduction, often down to hand motion or only light perception. Systemic symptoms, such as nausea and vomiting, may also occur. Signs include eyelid edema, mixed conjunctival hyperemia, corneal epithelial edema with a "microcystic" appearance under slit-lamp microscopy, and a subjective complaint of seeing "colored halos." Retrocorneal pigment deposits, an extremely shallow anterior chamber (with near-complete disappearance of the peripheral anterior chamber), and cloudy aqueous humor with possible fibrinous exudates may be observed. The pupil is moderately dilated, typically oval in shape, with absent light reflexes and sometimes posterior synechiae. The angle is completely closed, often exhibiting significant pigment deposition. Intraocular pressure frequently exceeds 50 mmHg. Optic nerve head changes, such as retinal arterial pulsation, disc edema, or retinal vascular occlusion, may be present, but severe corneal edema often prevents a clear fundus examination during an acute attack. After intraocular pressure is reduced, symptoms improve, vision recovers, but permanent damage to anterior segment structures may remain, including sectoral iris atrophy, pigment loss, posterior synechiae, a fixed and dilated pupil, and extensive peripheral anterior synechiae. Small localized lens opacities beneath the anterior capsule, known as glaucomatous flecks, may also be observed. The presence of these changes is diagnostic of a previous acute angle-closure glaucoma attack.
Intermittent Stage
After spontaneous resolution of minor attacks, the angle reopens fully or partially, and the trabecular meshwork remains intact. Intraocular pressure stabilizes without medication or with minimal use of miotic agents. The main diagnostic criteria for this stage include: (1) a clear history of minor attacks; (2) an open or partially open angle; and (3) stabilization of intraocular pressure within normal limits without medication or with minimal miotic treatment. Although theoretically, patients may enter the intermittent stage following an aggressively managed acute attack, in practice extensive peripheral anterior synechiae often make this unlikely.
Chronic Stage
After an acute attack or recurring minor attacks, extensive angle closure (usually exceeding 180°) occurs, leading to significant damage to trabecular meshwork function. Intraocular pressure is moderately elevated, and characteristic glaucomatous optic disc cupping and corresponding visual field defects become evident.
Absolute Stage
In cases of prolonged elevated intraocular pressure, severe damage to the ocular tissues, particularly the optic nerve, occurs. Vision deteriorates to a stage of no light perception, representing an irreversible late stage of the disease. Persistent elevated intraocular pressure or corneal degeneration may result in severe pain.
Diagnosis and Differential Diagnosis
The transient nature of minor attacks during the prodromal stage makes them difficult for clinicians to encounter directly. Diagnosis is often based on a history of transient attacks, along with characteristic findings such as a shallow anterior chamber and narrow angles. Minor attacks during the prodromal stage may sometimes be misdiagnosed as migraines. For patients with suspected cases, the dark room test may be used, in which the patient sits quietly in a dark room for 60 to 120 minutes while awake. Intraocular pressure is then measured under dim light conditions, and an increase of more than 8 mmHg compared to baseline is considered positive.
The symptoms and ocular signs of acute angle-closure glaucoma during a major attack are highly characteristic, and diagnosis is generally straightforward. Confirmation of angle closure by gonioscopy serves as a key diagnostic criterion. In some cases, it may be necessary to first reduce intraocular pressure with medications and apply topical glycerin to resolve corneal edema before an adequate gonioscopic evaluation of the angle can be performed. Compression gonioscopy can help differentiate between appositional contact and true synechial adhesion of the iris root to the trabecular meshwork. Following treatment, if intraocular pressure decreases but aqueous humor remains turbid to varying degrees, differentiation from acute iridocyclitis should be made. Key distinguishing features include:
- Pigmented deposits on the posterior cornea appear as brown pigment, rather than gray-white cellular deposits.
- The anterior chamber is extremely shallow.
- The pupil is moderately dilated rather than constricted.
- Iris changes may include segmental atrophy.
- Subcapsular glaucomatous flecks may be present on the anterior lens capsule.
- A history of minor attacks may be noted.
- The contralateral eye may exhibit anatomical features such as a shallow anterior chamber, iris thickening, and narrow angles.
Acute iridocyclitis, though also presenting with ocular pain, typically does not feature significant corneal epithelial edema, is often associated with lower intraocular pressure, demonstrates a constricted pupil, and shows signs of aqueous flare in the anterior chamber, with possible fibrinous exudates. These findings can aid in differentiation.
During a major attack, acute angle-closure glaucoma is often accompanied by systemic symptoms such as nausea, vomiting, and severe headache. These symptoms may overshadow the ocular pain and vision loss, leading to potential diagnostic confusion. It is necessary to consider and differentiate this condition from gastrointestinal disorders, cranial diseases, or migraines in clinical practice in order to avoid misdiagnosis and delays in treatment.
Chronic Angle-Closure Glaucoma
Chronic angle-closure glaucoma tends to develop at an earlier age compared to acute angle-closure glaucoma. The elevation in intraocular pressure associated with this condition also results from peripheral iris adhesions to the trabecular meshwork, impairing its function. However, in chronic angle-closure glaucoma, the peripheral adhesions progress gradually from localized areas to more diffuse involvement, leading to a progressive obstruction of the trabecular meshwork. The increase in intraocular pressure occurs slowly as the extent of angle closure expands.
Etiology
The anatomical risk factors for chronic angle-closure glaucoma include a relatively shallow anterior chamber and narrow angles compared to normal eyes. In some cases, the earliest peripheral iris adhesions develop at the site of iris surface elevations near the periphery, possibly due to the proximity of the iris to the trabecular meshwork, making contact more likely. Besides the pupillary block mechanism, chronic angle-closure glaucoma may also involve other non-pupillary block mechanisms such as peripheral iris crowding, which can contribute to angle closure. Ultrasound biomicroscopy (UBM) can differentiate between the pupillary block mechanism, characterized by iris bulging, and non-pupillary block mechanisms characterized by peripheral iris crowding. Factors contributing to progressive peripheral iris adhesions to the trabecular meshwork may vary, but a narrow angle serves as a fundamental condition for the disease.
Clinical Manifestations
Since angle closure and intraocular pressure elevation progress gradually in chronic angle-closure glaucoma, symptoms associated with abrupt intraocular pressure spikes are absent. The anterior segment appears relatively normal and does not raise suspicion in patients. However, the optic disc gradually atrophies due to sustained high intraocular pressure, forming glaucomatous cupping and leading to progressive visual field damage. In most cases, the condition is detected during routine eye examinations or at an advanced stage when patients notice visual field loss. Although the disease progresses chronically, resembling the course of primary open-angle glaucoma (POAG), the development of optic nerve damage tends to be faster in chronic angle-closure glaucoma.
Diagnosis
The diagnosis of chronic angle-closure glaucoma is based on the following points:
- The peripheral anterior chamber is shallow, while the depth of the central anterior chamber is slightly reduced or nearly normal, with minimal iris bulging.
- The angle is moderately narrow with varying degrees of peripheral iris adhesions to the trabecular meshwork.
- If both eyes are not affected simultaneously, the contralateral "healthy" eye may have normal intraocular pressure, optic disc appearance, and visual fields, but features such as narrow angles and localized peripheral iris adhesions may be observed.
- Moderate elevation in intraocular pressure is typically present.
- Glaucomatous cupping of the optic disc is evident in the fundus.
- Progressive visual field defects consistent with glaucoma are present to varying degrees.
Differentiation between chronic angle-closure glaucoma and POAG relies heavily on gonioscopy. While both conditions are characterized by elevated intraocular pressure, optic disc cupping, and visual field defects, POAG features a normal or deep anterior chamber and open angles despite elevated intraocular pressure.
Primary Open-Angle Glaucoma
Primary open-angle glaucoma (POAG) has an etiology that remains incompletely understood, though it is suspected to have a genetic basis. This condition is characterized by elevated intraocular pressure and persistently open angles, where aqueous outflow becomes obstructed at the level of the trabecular meshwork and Schlemm’s canal system. Histopathological studies suggest degeneration of collagen and elastic fibers within the trabecular meshwork, loss or proliferation of endothelial cells, thickening of the trabecular meshwork, narrowing or closure of meshwork pores, extracellular matrix deposits beneath the endothelium in Schlemm’s canal, and reduced vacuolization of endothelial cells lining Schlemm’s canal.
Clinical Manifestations
Symptoms
The onset is insidious. Most patients do not exhibit noticeable symptoms and may remain asymptomatic until significant visual function has been compromised in advanced stages. Only a small number of patients experience transient blurry vision ("foggy vision") or ocular fullness during episodes of elevated intraocular pressure.
Intraocular Pressure
In the early stages, intraocular pressure may exhibit instability and occasionally fall within the normal range. Measurement over a 24-hour period is helpful in detecting peak intraocular pressure and large fluctuations. Overall intraocular pressure tends to be slightly higher than normal and increases steadily as the disease progresses.
Anterior Segment
The anterior chamber is typically normal or slightly deeper in depth, and the iris remains flat. The angles are consistently open. Except in cases where asymmetric optic nerve damage between the eyes leads to relative afferent pupillary defects, the anterior segment usually lacks notable abnormalities.
Fundus
The major fundus changes associated with glaucomatous optic disc damage include:
- Progressive enlargement and deepening of optic disc cupping.
- Focal thinning of the neuroretinal rim, particularly in the superior and inferior regions, with increases in the vertical cup-to-disc ratio (C/D value) or the formation of notching.
- Asymmetry in optic disc cupping between the two eyes, with a C/D difference of >0.2.
- Superficial linear hemorrhages on or around the optic disc.
- Retinal nerve fiber layer defects.
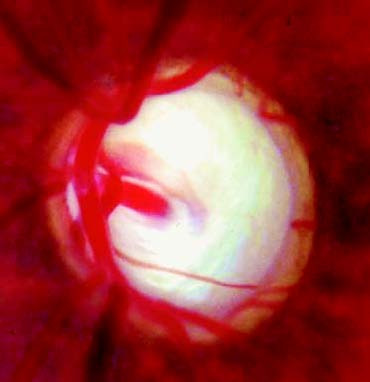
Figure 6 Glaucomatous optic disc cupping (glaucomatous cup)
Visual Function
Changes in visual function, particularly visual field defects, serve as critical indicators for diagnosing glaucoma and evaluating disease progression. The visual field defects in glaucoma are characterized by a pattern linked to the anatomical distribution and structure of the retinal nerve fiber layer, as well as the characteristics of glaucomatous damage to the optic disc and retina. Early visual field changes often manifest as isolated paracentral scotomas or nasal step defects. Paracentral scotomas are typically found within 5°–25° of the visual field, near the physiological blind spot. As the disease progresses, these defects enlarge, deepen, and merge, extending toward the nasal aspect to form arcuate scotomas. Peripheral visual fields gradually constrict centripetally, fusing with paracentral defects to create quadrantic or hemifield loss. In advanced stages, only a "tunnel vision" or a temporal field island may survive. Automated perimetry with quantitative assessment of light thresholds can detect early glaucomatous changes, such as diffuse or localized increases in threshold values and greater variability in sensitivity.
Although central visual acuity is generally preserved even in late-stage glaucoma, with some patients retaining acuities of 1.0 despite severe tubular field constriction, recent studies suggest that POAG also affects macular function. This includes acquired color vision deficits, reduced contrast sensitivity, and abnormalities in certain electrophysiological tests such as pattern electroretinography and visual-evoked potentials. However, these parameters are less specific compared to visual field changes.
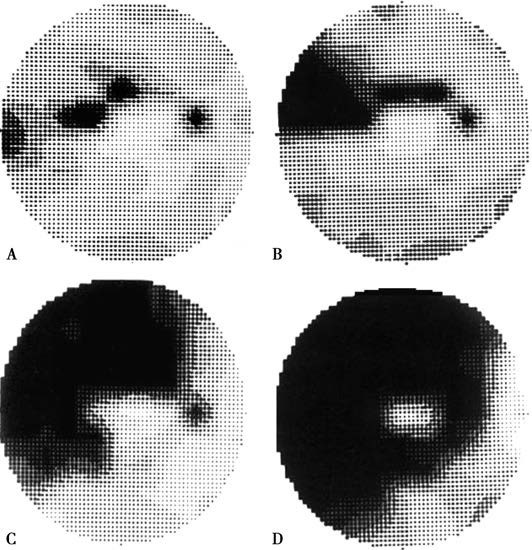
Figure 7 Glaucomatous visual field defects
POAG typically affects both eyes but often demonstrates asymmetric progression, with differences in intraocular pressure, optic disc appearance, visual field changes, and pupillary light reflex responses between the two eyes.
Diagnosis
Primary open-angle glaucoma (POAG) typically lacks noticeable symptoms, making it highly prone to being missed in the early stages. Diagnosis often relies heavily on population health screening. The primary diagnostic criteria are as follows:
Elevated Intraocular Pressure
In the early stages of the disease, intraocular pressure (IOP) is not consistently elevated. Approximately 50% of glaucoma patients have single IOP measurements below 22 mmHg. Therefore, a normal IOP reading on one or two occasions does not rule out the possibility of glaucoma. Measuring IOP over a 24-hour period aids in identifying peak IOP values and variations in pressure. In patients with lower scleral rigidity, such as those with high myopia, the IOP measured using a standard Schiötz indentation tonometer may underestimate the true IOP. In such cases, an applanation tonometer should be used, or the measurements should be corrected to better reflect the actual IOP.
Optic Disc Damage
Glaucomatous changes in the optic disc include progressive deepening and enlargement of the optic cup, with neuroretinal rim thinning that is most pronounced in the superior and inferior regions or in localized areas. Optic disc hemorrhages and defects in the retinal nerve fiber layer are also characteristic of glaucomatous optic neuropathy. Additionally, asymmetry in optic disc morphology between the two eyes, such as a difference in the vertical cup-to-disc ratio (C/D) greater than 0.2, is diagnostically significant.
Visual Field Defects
Reproducible paracentral scotomas or nasal step defects are often indicative of early glaucomatous visual field damage. Early visual field defects are more easily detected using supra-threshold static perimetry with a Goldmann perimeter or threshold testing with an automated perimetry system. A strong correlation exists between optic disc damage and visual field defects, and when these findings align, they provide mutual confirmation.
If two of the three main diagnostic criteria—elevated IOP, optic disc damage, or visual field defects—are met, while gonioscopy confirms an open angle, a diagnosis of POAG can be established. Additional supportive findings include reduced aqueous humor outflow facility, relative afferent pupillary defects, acquired color vision abnormalities, decreased contrast sensitivity, abnormalities in certain visual electrophysiological tests, and a positive family history of glaucoma. These findings offer further reference value in diagnosing open-angle glaucoma.
When characteristic glaucomatous optic disc and visual field damage are present, but IOP remains within the statistically normal range, the condition may be diagnosed as normal tension glaucoma (NTG). NTG is generally thought to result from abnormalities within the optic nerve itself, such as insufficient blood flow or reduced tolerance to IOP, which can lead to optic nerve damage even at normal pressure levels. Compared to POAG, patients with NTG are more likely to have vascular spasm-related conditions such as migraines, Raynaud's phenomenon, or ischemic vascular diseases. NTG is also associated with features such as optic disc hemorrhages, inferior or inferotemporal notching, and peripapillary atrophy. Visual field defects in NTG are often more localized and closer to the fixation point.
Differential diagnosis of NTG should consider ischemic optic neuropathy, congenital abnormalities of the optic nerve, and optic atrophy caused by intracranial space-occupying lesions. Recent findings suggest that low cerebrospinal fluid pressure may also be a risk factor for NTG. Low intracranial pressure increases the translaminar pressure gradient, which may lead to optic nerve damage. Additionally, in some POAG patients with thin central corneas, IOP readings may underestimate actual pressure, resulting in misclassification as NTG. In certain POAG patients, daytime IOP measurements may fall within the normal range, but 24-hour IOP monitoring reveals nocturnal IOP spikes above normal levels, potentially leading to a misdiagnosis of NTG.
Treatment for NTG includes neuroprotective therapy and further lowering IOP through medications or surgical interventions.
Treatment of Primary Glaucoma
The goal of glaucoma treatment is to preserve visual function. Treatments primarily aim to:
Lower Intraocular Pressure (IOP)
Since IOP is a relatively controllable risk factor, current glaucoma treatment strategies focus on lowering IOP through medications or surgical interventions. The target IOP is defined as the pressure level at which further damage to the optic nerve is minimized. This target IOP varies depending on the individual and the specific eye involved. Patients with severe optic nerve damage typically have a lower tolerance to IOP, leading to a lower target IOP. In advanced cases, IOP must be reduced to an even lower level to prevent further progression of the disease. Target IOP is also influenced by the level of IOP present when optic nerve damage occurred, the rate of glaucoma progression, the patient’s age, and anticipated life expectancy. In addition to peak IOP, large diurnal IOP fluctuations are also considered a risk factor for disease progression, making 24-hour IOP measurement an important tool for monitoring IOP control. However, since IOP is not the sole risk factor for glaucoma, some patients may experience progressive optic nerve atrophy and visual field loss even after IOP is effectively managed. As such, the target IOP represents only a relatively safe pressure level.
Neuroprotective Therapy
Neuroprotection is aimed at preserving the optic nerve by improving its blood supply and mitigating retinal ganglion cell apoptosis.
Common IOP-Lowering Medications
Medications reduce IOP through three primary mechanisms: increasing aqueous humor outflow, suppressing aqueous humor production, or reducing intraocular volume. Among these, increasing aqueous humor outflow aligns most closely with the physiological mechanisms for maintaining normal aqueous dynamics.
Parasympathomimetics (Miotic Agents)
Pilocarpine, in concentrations of 1%–4%, is commonly used as eye drops applied 3–4 times per day, or as a 4% gel applied once at night. Pilocarpine directly stimulates the pupillary sphincter muscle to induce miosis and increase iris tension, thereby alleviating blockage of the trabecular meshwork by the peripheral iris. This allows reopening of the anterior chamber angle, making pilocarpine the first-line treatment for angle-closure glaucoma. In the treatment of open-angle glaucoma, pilocarpine lowers IOP by stimulating ciliary muscle contraction, which pulls on the scleral spur and trabecular meshwork, reducing outflow resistance and increasing aqueous humor drainage.
Beta-Adrenergic Receptor Blockers
Frequently used options include timolol (0.25%–0.5%), levobunolol (0.25%–0.5%), and betaxolol (0.25%–0.5%) in the form of eye drops applied once or twice daily. Beta blockers reduce IOP by suppressing aqueous humor production. These medications have minimal effects on pupil size and accommodative function, but their efficacy in lowering IOP may diminish with prolonged use.
Adrenergic Receptor Agonists:
α2 adrenergic receptor agonists, such as 0.2% brimonidine, selectively stimulate α2 receptors to reduce aqueous humor production and facilitate aqueous outflow through the uveoscleral pathway. Brimonidine has minimal effects on α1 receptors, avoiding pupil dilation, and has no significant impact on cardiac or pulmonary function.
Prostaglandin Analogues
Commonly available agents include 0.005% latanoprost, 0.004% travoprost, and 0.03% bimatoprost. These medications lower IOP by increasing the outflow of aqueous humor through the uveoscleral pathway without reducing aqueous humor production. Once-daily evening dosing can achieve a 20%–40% reduction in IOP. These drugs do not affect cardiovascular or pulmonary function; side effects primarily include brief local burning, stinging, itching, and conjunctival hyperemia after application. Long-term use may cause increased iris pigmentation, eyelash growth, and hyperpigmentation of periorbital skin.
Carbonic Anhydrase Inhibitors
Acetazolamide, dosed at 250 mg per tablet, reduces IOP by decreasing aqueous humor production and is often used as an adjunct to topical therapy. A reduced dosage, such as 125 mg twice daily or 62.5 mg three times daily, is recommended to minimize side effects. Prolonged use may lead to symptoms such as numbness in the lips, face, and extremities, systemic malaise, renal colic, or hematuria, making long-term use unsuitable. Topical formulations, such as 1% brinzolamide, have been developed and offer slightly less IOP reduction than systemic acetazolamide but have fewer systemic side effects.
Hyperosmotic Agents
Common options include 50% glycerin (administered orally at 2–3 mL/kg) and 20% mannitol (administered intravenously at a rapid infusion rate of 1–2 g/kg). These agents temporarily increase plasma osmolarity, drawing fluid from ocular tissues, particularly the vitreous body, into the bloodstream to reduce intraocular volume and rapidly lower IOP. The IOP-lowering effect typically lasts 2–3 hours. Hyperosmotic agents are primarily used for acute angle-closure glaucoma attacks and secondary glaucoma with acute IOP elevation. After administration, reduced intracranial pressure may cause symptoms such as headache and nausea in some patients, necessitating rest in a supine position. Since glycerin is metabolized into glucose, caution is advised in diabetic patients.
Common Glaucoma Surgeries
Surgeries to Relieve Pupillary Blockage
Procedures such as peripheral iridectomy and laser iridotomy are commonly performed to address pupillary block. The primary principle of these surgeries involves removing or creating an opening in the peripheral iris to establish a pathway of communication between the anterior and posterior chambers, effectively relieving pupillary block. Postoperatively, the pressure in the anterior and posterior chambers achieves equilibrium, which is often effective in preventing the recurrence of angle-closure glaucoma. Peripheral iridectomy and laser iridotomy are primarily indicated for early-stage primary and secondary angle-closure glaucoma caused by pupillary block, provided there is no extensive synechial closure of the anterior chamber angle.
Surgeries to Reduce Trabecular Meshwork Resistance
Examples include goniotomy, trabeculotomy, and selective laser trabeculoplasty (SLT). Goniotomy and trabeculotomy involve cutting into underdeveloped or insufficiently permeable trabecular meshwork from the inner or outer surface, respectively, to allow aqueous humor to drain through normal physiological pathways into the venous system. These types of surgeries can often achieve a curative effect for primary congenital glaucoma. SLT employs laser therapy to activate cellular components within the trabecular meshwork, inducing the production of matrix metalloproteinases that degrade the extracellular matrix, thereby reducing outflow resistance and lowering IOP. SLT is mainly used for early primary open-angle glaucoma (POAG) or as adjunctive therapy in POAG patients whose IOP is inadequately controlled with medications. While the long-term efficacy of SLT in lowering IOP is limited, the procedure can be repeated.
Surgical Creation of Aqueous Outflow Pathways (Filtering Surgeries)
Procedures such as trabeculectomy, nonpenetrating trabecular surgery, laser sclerostomy, and implantation of aqueous drainage devices fall into this category. These filtering procedures generally involve the removal of a portion of the corneoscleral trabecular tissue to create a fistula. This fistula allows aqueous humor to drain into the subconjunctival space, where it is absorbed by capillaries and lymphatic vessels within the conjunctival tissues, ultimately reducing IOP. Such procedures are primarily indicated for POAG and angle-closure glaucoma with extensive anterior chamber angle adhesions.
Surgeries to Reduce Aqueous Humor Production
Techniques in this category include cyclocryosurgery, cyclodiathermy, and cyclophotocoagulation. These procedures involve the destruction of the ciliary body and its associated vasculature through cryotherapy, diathermy, or laser photocoagulation, thus reducing aqueous humor production to achieve IOP reduction and symptom control. Ciliary body destruction procedures are typically reserved for patients with absolute glaucoma accompanied by significant pain.
Combined Glaucoma and Cataract Surgery
In angle-closure glaucoma, lens swelling and anterior displacement of the lens are major factors contributing to pupillary block. Removal of the lens addresses these mechanical factors, effectively preventing the occurrence of angle-closure glaucoma from its underlying mechanism.
Combined glaucoma and cataract surgery is recommended for glaucoma patients with progressive moderate-to-severe optic nerve damage, as well as for those whose IOP is maintained at normal or borderline levels with two or more anti-glaucoma medications. Additional indications include patients with poor compliance or limited access to follow-up care, those unable to tolerate or use glaucoma medications, and cases where single-stage surgery is necessary due to urgent visual needs. This approach is also suitable for patients with previously dysfunctional filtering blebs and uncontrolled IOP. Combined surgery can reduce the risk of transient postoperative IOP spikes often associated with standalone cataract extraction, which may otherwise result in irreversible visual function impairment.
Treatment of Primary Angle-Closure Glaucoma (PACG)
The elevated intraocular pressure (IOP) in PACG results from the blockage of aqueous humor outflow by the peripheral iris. The primary objective of treatment involves alleviating pupillary block or reshaping the peripheral iris to widen the anterior chamber angle, thereby preventing contact and adhesion between the peripheral iris and the aqueous outflow pathways.
The fundamental treatment principle for acute angle-closure glaucoma involves reopening the anterior chamber angle or establishing a new drainage pathway using medications, laser interventions, or surgery. Preoperative comprehensive medical therapy plays a crucial role in constricting the pupil, opening the anterior chamber angle, rapidly controlling IOP, and minimizing tissue damage. Surgical outcomes tend to be more favorable after adequately reducing IOP and controlling inflammatory responses.
Pupil Constriction
During the prodromal stage or minor attacks, 1% pilocarpine eye drops are administered every 30 minutes for 2–3 applications, typically achieving effective pupil constriction and IOP reduction. In cases of an acute severe attack, pilocarpine is applied every 5 minutes for three applications, followed by every 30 minutes for four applications, and thereafter once every hour. If the sphincter muscle of the pupil has not been impaired, significant constriction can generally be observed within 3–4 hours, allowing the dose to be reduced to four times daily. However, if IOP is extremely elevated, the sphincter muscle of the pupil becomes paralyzed, or ischemic necrosis of the iris occurs, miotics may become ineffective. Better results can often be achieved by using systemic IOP-lowering medications before applying miotics. To prevent systemic toxicity from nasal mucosal absorption, pressing a cotton ball against the lacrimal sac area for several minutes after each application of high-concentration miotics is recommended.
Combination Therapy
During acute attacks, in addition to local application of miotics, combination therapy is often required, such as systemic administration of hyperosmotic agents and carbonic anhydrase inhibitors, as well as local application of beta-blockers for rapid IOP control.
Adjunctive Treatments
Patients with severe systemic symptoms may require antiemetics, sedatives, or hypnotic drugs for symptom management. The use of topical corticosteroids can help reduce conjunctival hyperemia and inflammation of the iris.
Surgical Treatment
After the resolution of acute angle-closure glaucoma, IOP often remains stable at lower levels for several weeks due to ciliary body ischemia and decreased aqueous humor production. However, IOP at this stage is not a reliable indicator of angle function. It is essential to communicate to patients that reducing IOP with medication does not conclude treatment and that further surgical intervention is necessary.
Prior to surgery, careful examination of the anterior chamber angle is performed, along with frequent IOP measurement while using only pilocarpine drops. If the anterior chamber angle remains open or if synechial closure involves less than one-third of the angle circumference and IOP stabilizes below 21 mmHg, procedures such as peripheral iridectomy, laser iridotomy, or combined cataract extraction with goniosynechialysis are indicated. These procedures aim to establish communication between the anterior and posterior chambers, alleviate pupillary block, balance the pressures in the two chambers, reduce iris bulging, and widen the anterior chamber angle, preventing further contact between the peripheral iris and trabecular meshwork.
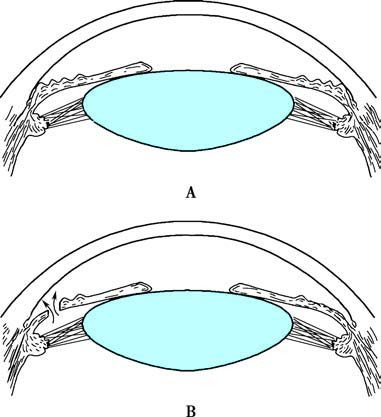
Figure 8 Principle of peripheral iridectomy for acute angle-closure glaucoma
A. Schematic representation of anterior chamber angle closure in acute angle-closure glaucoma.
B. After peripheral iridectomy, the iris bulge is resolved, and the anterior chamber angle widens.
If there is extensive synechial closure of the anterior chamber angle and IOP exceeds 21 mmHg despite pilocarpine use, this indicates permanent damage to the trabecular meshwork. In such cases, filtering surgeries should be performed.
In rare clinical cases, despite combination therapy, IOP remains uncontrollably high. In such situations, after medical treatment reduces corneal edema, procedures such as laser peripheral iridoplasty and laser iridotomy may be considered to quickly alleviate pupillary block. If laser iridotomy is not feasible, anterior chamber paracentesis may be attempted to prevent significant damage to the optic nerve caused by persistently elevated IOP.
At the preclinical stage, if untreated, 40–80% of cases may progress to acute attacks within 5–10 years. Long-term use of pilocarpine does not reliably prevent acute attacks, so early prophylactic peripheral iridectomy or laser iridotomy is recommended for preclinical patients with iris bulging, shallow anterior chambers, and narrow angles.
The treatment goal for chronic angle-closure glaucoma (CACG) is also to manage IOP using medications, laser therapy, or surgical interventions to protect the optic nerve. Since pupillary block is not a prominent factor in CACG, peripheral iridectomy is less targeted than in acute angle-closure glaucoma. Nevertheless, peripheral iridectomy can be beneficial in preventing pupillary block caused by prolonged pilocarpine use and can, to some extent, help prevent or slow further synechial closure of the anterior chamber angle. Peripheral iridectomy is therefore suitable for early-stage cases with pupillary block, limited synechial closure of the angle, and successful IOP control with miotics alone.
For cases of CACG not involving pupillary block mechanisms, peripheral iridectomy alone is often insufficient to prevent progressive closure of the anterior chamber angle. Argon laser peripheral iridoplasty is preferred in such cases to widen the angle.
For patients with extensive synechial angle closure, uncontrolled IOP despite miotic therapy, or significant optic nerve damage, filtering surgeries are required.
Treatment of Primary Open-Angle Glaucoma (POAG)
Medical Treatment
The primary cause of elevated intraocular pressure (IOP) in POAG is reduced trabecular meshwork permeability. Medications that increase aqueous humor outflow through the trabecular meshwork, such as miotics, offer etiological treatment but are limited in POAG due to their side effects. Although reducing aqueous humor production is not a causal treatment, inhibitors of aqueous humor formation are more widely used in clinical practice because of their relatively mild side effects. Prostaglandin analogs, which enhance aqueous humor outflow through the uveoscleral pathway, are currently an important pharmacological choice for treating POAG.
If 1–2 topical medications can effectively lower IOP to a safe level and the patient is able to adhere to treatment and attend regular follow-ups, medication therapy typically serves as the initial approach. In the absence of contraindications, current glaucoma guidelines recommend prostaglandin analogs as the first-line treatment. If one medication does not provide adequate IOP control, a different medication may be considered. For cases where monotherapy fails to lower IOP to a safe level, combination therapy is utilized, with each medication administered at least 10 minutes apart. Post-application techniques, such as applying pressure to the lacrimal sac and gently closing the eyelids for 1–2 minutes, may help maintain local drug concentrations and reduce systemic absorption.
Laser Treatment
If medical therapy proves inadequate, selective laser trabeculoplasty (SLT) is often considered as an alternative.
Filtering Surgery
Trabeculectomy is the most frequently performed surgical procedure for POAG. Surgery is generally indicated in cases where medical therapy is ineffective, poorly tolerated, or not feasible. Recently, there has been advocacy for initiating filtering surgery as a first-line treatment in cases where a definitive diagnosis is made and there is already significant optic disc or visual field damage. It is suggested that early surgical intervention may yield better outcomes compared to surgeries performed after the failure of long-term medical therapy.
Neuroprotective Treatment for the Optic Nerve
Glaucoma is characterized by the progressive death of retinal ganglion cells. Studies have shown that the mechanism underlying ganglion cell death involves apoptosis. Elevated IOP or ischemia of the optic nerve acts as the trigger for the disease, while factors such as free radicals, depletion of neurotrophic factors, and increased concentrations of the excitatory neurotoxin glutamate may serve as inducers of ganglion cell apoptosis. Therefore, in addition to lowering IOP, comprehensive glaucoma management should also encompass neuroprotective therapies for the optic nerve.
Current research efforts are focused on neutralizing apoptotic triggers, developing exogenous and endogenous neurotrophic factors, genetic therapy, and promoting neuronal regeneration or transplantation to control ganglion cell apoptosis and achieve optic nerve preservation.
Calcium channel blockers, glutamate antagonists, neurotrophic factors, and antioxidants (e.g., vitamins C and E) have shown potential in providing different degrees of neuroprotection to the optic nerve. β1 receptor blocker betaxolol, in addition to lowering IOP, has been found to increase blood flow to the optic nerve. Similarly, the α2 receptor agonist brimonidine has demonstrated some neuroprotective effects.