Acanthamoeba keratitis, caused by Acanthamoeba infection, is a severe form of keratitis that poses a serious threat to vision. It is often characterized as a chronic, progressive corneal ulcer with a disease course that can last for several months.
Etiology
More than 50 species of Acanthamoeba have been identified, which are widely distributed in soil, freshwater, seawater, swimming pools, grains, and livestock. Acanthamoeba exists in two forms: active trophozoites and dormant cysts. Among these species, eight are associated with human infections, with Acanthamoeba castellanii being the most common. The morphological plasticity of Acanthamoeba in different environments makes it difficult to further classify and identify the pathogen based on morphological characteristics alone. Techniques such as immunofluorescence, enzymatic profiling, and genetic testing are commonly employed.
Clinical Presentation
Approximately 90% of cases are related to contact lens use. Corneal trauma and exposure to soil or water contaminated with Acanthamoeba are also common causes. Most infections are unilateral, with symptoms including photophobia, tearing, severe eye pain, and reduced visual acuity. The disease often has a protracted course lasting several months. Clinical manifestations are diverse and can be easily confused with herpes simplex keratitis or fungal keratitis. Additionally, the clinical presentation varies at different stages of the disease.
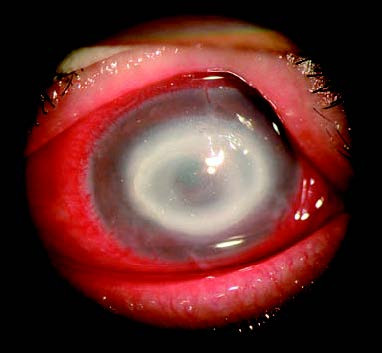
Figure 1 Acanthamoeba keratitis
A central discoid corneal ulcer is observed, with annular infiltrates surrounding the lesion peripherally.
In the early stages of infection, findings may include epithelial haze, microcystic edema, or pseudo-dendritic lesions, with the epithelium remaining intact. A minority of patients (2.0%–6.6%) may present with the characteristic feature of radial keratoneuritis. As the disease progresses, annular infiltrates may appear in the central or paracentral cornea, often accompanied by epithelial defects. In some cases, there may be central disciform lesions, stromal edema and thickening, or pinpoint to patchy opacities. In advanced stages, tissue protease and collagenase release result in stromal dissolution, abscess formation, corneal ulceration, or even perforation, although anterior chamber reactions are rare.
Acanthamoeba sclerokeratitis is a severe complication of Acanthamoeba keratitis, with an incidence of 14%–16%. It is characterized by diffuse anterior scleritis, occasionally accompanied by posterior scleritis or optic neuritis. Symptoms are generally severe, treatment is challenging, and the pathogenesis remains unclear.
Diagnosis
Diagnosis is based on identifying Acanthamoeba trophozoites or cysts in corneal scrapings through staining or culturing Acanthamoeba from corneal specimens. Corneal biopsy can be performed when necessary. In vivo diagnosis can also be supported by confocal microscopy.
Treatment
Early diagnosis and treatment significantly improve the prognosis of Acanthamoeba keratitis. Early-stage treatment may involve debridement of the affected corneal epithelium. Medications commonly used include aminoglycosides, biguanides, bis-biguanides, diamidines, and imidazoles, with combination therapy being a standard approach. Reports have documented successful treatment using 0.02%–0.1% chlorhexidine, 0.01%–0.02% polyhexamethylene biguanide (PHMB), 0.15% dibromopropamidine isethionate, or 1% miconazole. Oral itraconazole or ketoconazole may also be used as adjunctive therapy.
Treatment durations tend to be prolonged. Initially, topical medications are often applied hourly, and as symptoms improve, the frequency is gradually reduced to 4–6 times daily, with treatment lasting for more than 4 months until infection is fully controlled and all pathogens are eradicated. Interrupting treatment prematurely may cause recurrence and exacerbate the condition.
The use of corticosteroids is generally avoided due to the risk of exacerbating the disease.
When medical therapy proves ineffective, ulcers fail to heal, or perforation occurs, corneal transplantation may be considered.
After successful treatment, if corneal scarring significantly impairs vision, penetrating keratoplasty may be performed. Postoperative medical therapy is required to reduce the risk of recurrence. Treatment outcomes are generally poor when the infection spreads to the sclera, as both medical and surgical therapies become less effective.