Fungal keratitis is an infectious corneal inflammation caused by pathogenic fungi. This condition is associated with a high risk of blindness and occurs more frequently in warm, humid climates. Its incidence is notably higher in tropical and subtropical regions, particularly in locations near the equator.
Etiology
A variety of fungi can cause corneal infections; however, the majority of cases are attributed to four main types of fungi: Fusarium species (Fusarium solani, Fusarium oxysporum), Aspergillus species (Aspergillus fumigatus), Curvularia species (Curvularia lunata), and Candida species (Candida albicans). The first three belong to the filamentous fungi category and are more commonly associated with corneal infections among individuals who engage in agricultural or outdoor work in moist environments. Trauma, especially plant-related injuries, is the most common predisposing factor. Other contributing factors include prolonged use of corticosteroids or antibiotics resulting in altered ocular surface immunity or microbial imbalance, allergic conjunctivitis, contact lens wear, corneal transplantation, or refractive corneal surgery.
Candida species, which are yeasts, often cause infections secondary to pre-existing ocular surface diseases (e.g., dry eye, lagophthalmos, viral keratitis) or systemic immune suppression in patients with conditions such as diabetes or immunosuppression.
Clinical Presentation
A history of plant-related corneal trauma (e.g., tree branches, sugarcane leaves, straw) or prolonged corticosteroid and antibiotic use is frequently observed. The onset is insidious, with a subacute progression. Symptoms tend to be mild but are accompanied by visual impairment. Corneal infiltrates appear as white or milky-white dense lesions with a dull surface resembling toothpaste or plaque. Surrounding the ulcer, a shallow trench formed by stromal dissolution or an immune ring due to antigen-antibody reactions may be observed. Occasionally, "pseudopodia" or satellite infiltrates may be seen adjacent to the main lesion, and corneal endothelial plaques may occur posteriorly. Hypopyon is grayish-white, thick, or paste-like in appearance.
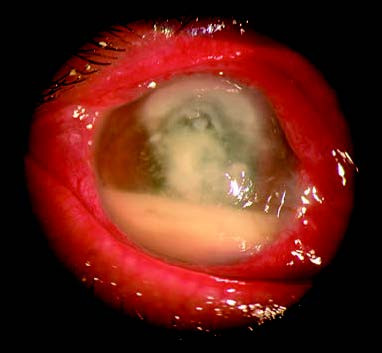
Figure 1 Fungal corneal ulcer
The central corneal infiltrate appears milky white, dense, with a dull and dry surface resembling a toothpaste-like texture. Hypopyon in the anterior chamber is grayish-white, thick, or paste-like in appearance.
Certain fungal species exhibit specific patterns of keratitis. For example, keratitis caused by Fusarium solani progresses rapidly and tends to be severe, often infiltrating deeper corneal tissues and causing corneal perforation within weeks. Ocular complications, including malignant glaucoma, may arise due to fungal proliferation and inflammatory responses in the posterior iris. Aspergillus-associated keratitis progresses more slowly and is generally less severe, with relatively better response to medical treatment. Curvularia-induced keratitis is typically characterized by feather-like infiltrates confined to the superficial stromal layers and progresses slowly. This type responds well to natamycin and has a lower incidence of complications such as corneal perforation.
Filamentous fungi have strong invasive potential, with hyphae capable of penetrating deep stromal layers to invade Descemet's membrane and even entering the anterior chamber to affect the iris and other intraocular tissues. Intraocular fungal infections are primarily located in the posterior chamber, particularly along the peripheral region between the iris and lens. This can result in persistent fungal iridocyclitis, pupillary membrane occlusion, secondary glaucoma, complicated cataracts, or fungal endophthalmitis. Once fungi invade the anterior chamber, the disease becomes difficult to control.
Diagnosis
A preliminary diagnosis can be made based on a history of plant-related trauma and the characteristic features of corneal lesions. Corneal confocal microscopy, as a non-invasive examination technique, can directly detect fungal pathogens within the lesion in the early stages of the disease. The presence of hyphae detected by confocal microscopy or fungi and hyphae identified through laboratory testing confirms the diagnosis.
Common rapid diagnostic techniques include corneal scraping followed by Gram staining, Giemsa staining, 10%–20% potassium hydroxide (KOH) wet mount, lactophenol cotton blue (LPCB) staining, methenamine silver staining, calcofluor white staining, and periodic acid-Schiff (PAS) staining.
Fungal culture can utilize media such as blood agar, chocolate agar, potato dextrose agar, and Sabouraud agar, with incubation at 30–37°C. Fungal growth is usually visible within 3–4 days, but culture should be maintained for 4–6 weeks. Positive cultures can undergo microscopic examination and susceptibility testing. Additionally, advanced techniques such as immunofluorescence staining, electron microscopy, and PCR are also employed for the diagnosis of fungal keratitis.
Treatment
Antifungal medications are administered locally for treatment. These include polyenes (such as 0.15% amphotericin B or 5% natamycin eye drops), imidazoles (such as 0.5% miconazole eye drops), or pyrimidines (such as 1% flucytosine eye drops). Currently, 0.15% amphotericin B and 5% natamycin eye drops are considered first-line medications for fungal keratitis. For filamentous fungi, 5% natamycin or voriconazole is preferred, while for yeast infections, 0.15% amphotericin B, 2% fluconazole, or 5% natamycin may be used. A combination of antifungal medications provides a synergistic effect, reducing the required dosage of individual drugs and minimizing side effects. Common combination regimens include natamycin with amphotericin B or fluconazole. Rifampin can enhance the antifungal activity of amphotericin B and is also frequently used in combination.
Antifungal medications are initially applied topically every 0.5–1 hour to increase drug concentration at the lesion site, with nighttime application of antifungal ointments. As the infection becomes controlled, the frequency of administration is gradually reduced. In severe cases, systemic antifungal therapy may be needed, including oral administration of fluconazole, ketoconazole, itraconazole, or voriconazole, or intravenous infusion of miconazole, fluconazole, or voriconazole. Systemic treatment requires close monitoring for drug-related side effects, particularly hepatotoxicity. Antifungal medications tend to work slowly, and clinical signs must be observed carefully during treatment to evaluate efficacy. Indicators of effective treatment may include pain relief, reduced size of infiltrates, disappearance of satellite lesions, and smooth and rounded ulcer margins. Even when treatment shows effectiveness, antifungal therapy is typically continued for at least six weeks. Monitoring for ocular surface toxicity, such as conjunctival hyperemia, edema, or punctate corneal epithelial erosion, is important during treatment.
Recent studies have shown that immunosuppressants like cyclosporine and tacrolimus (FK506) can inhibit the growth of Fusarium solani, Fusarium oxysporum, and Aspergillus fumigatus. Although they are ineffective against Candida albicans, combining these agents with fluconazole can enhance antifungal effects against yeast infections. Rifampin, a macrolide antibiotic, has shown efficacy in treating yeast and Cryptococcus infections.
For managing concurrent iridocyclitis, 1% atropine eye drops or ointment is utilized to induce mydriasis. Glucocorticoids are not recommended.
In severe cases, surgical intervention may be necessary. Surgical options include debridement, conjunctival flap coverage, and keratoplasty. Early debridement facilitates drug penetration into the corneal stroma, increases drug concentration at the lesion site, and aids in pathogen removal. Conjunctival flap coverage leverages the conjunctival blood supply to deliver anti-inflammatory factors to the lesion, thereby achieving fungal suppression. For cases where medical treatment is ineffective and the lesion has not invaded the deep stroma, lamellar keratoplasty may be considered. When corneal ulceration is imminent or perforation has already occurred, penetrating keratoplasty may be required. Postoperative antifungal therapy is crucial to prevent recurrence of infection.