Bacterial keratitis refers to inflammation of the cornea caused by bacterial infection, leading to corneal epithelial defects and stromal necrosis. It is also known as bacterial corneal ulcer. The condition is often severe, and without effective treatment, it can result in corneal ulceration and perforation, or even intraocular infections. Even when the condition is controlled, it may leave significant sequelae such as extensive corneal scarring, corneal neovascularization, or corneal lipid degeneration, which can seriously impair vision and, in some cases, lead to blindness.
Etiology
A wide variety of bacteria can cause bacterial keratitis. The most common pathogens include Staphylococcus, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Escherichia coli. Staphylococcus species remain the most frequent causative agent of bacterial keratitis, with an increasing detection rate reported in some Western countries. Pseudomonas aeruginosa is another major pathogen, commonly associated with the use of contact lenses. While the incidence of Streptococcus pneumoniae-induced keratitis has declined in developed countries, it remains prevalent in developing regions. The spectrum of causative bacteria can vary by region, time period, and environmental factors such as the distribution of normal conjunctival flora, local temperature and humidity, and climate.
Risk factors for bacterial keratitis include both local and systemic elements. The most common local factors are corneal trauma or foreign body removal, often associated with poor aseptic techniques or the use of contaminated topical anesthetics or fluorescein. Contact lens use and chronic dacryocystitis are also significant risk factors. Other local factors include dry eye and prolonged use of corticosteroids in the ocular area.
Systemic factors include aging, Vitamin A deficiency, diabetes, and immunodeficiency, all of which can reduce the body's resistance to pathogens or make the cornea more susceptible to infections.
Clinical Manifestations
Severe bacterial keratitis typically has an acute onset, presenting with symptoms such as photophobia, tearing, eye pain, vision disturbances, and blepharospasm. The eyelids and bulbar conjunctiva often become edematous, with ciliary or mixed conjunctival hyperemia. In the early stages, corneal epithelial ulceration is observed, with an underlying dense infiltration area featuring indistinct borders and surrounding tissue edema. The infiltration progresses rapidly, leading to stromal dissolution and ulcer formation, often accompanied by purulent or mucopurulent discharge on the ulcer surface and in the conjunctival sac. Hypopyon of varying degrees may occur. Infections caused by Nontuberculous Mycobacteria or other low-virulence bacteria tend to have a more insidious onset with milder symptoms.
Severe bacterial keratitis is often caused by pathogens such as Staphylococcus aureus, Streptococcus pneumoniae, Beta-hemolytic streptococci, or Pseudomonas aeruginosa.
Staphylococcus aureus keratitis typically presents as a rounded or oval focal abscess with surrounding grayish-white infiltrative areas and well-defined borders, often arising in previously compromised corneas. Such cases might involve conditions like bullous keratopathy, Herpes simplex keratitis, keratoconjunctivitis sicca, ocular lupus erythematosus, or allergic keratoconjunctivitis. Without effective treatment, severe stromal abscess or corneal perforation may occur.
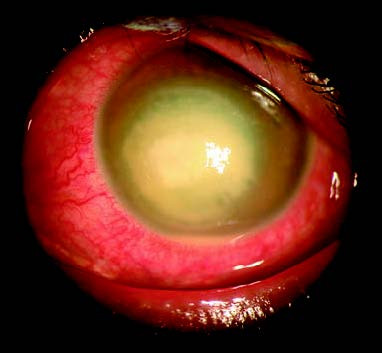
Figure 1 Staphylococcal corneal ulcer
A nearly circular focal abscess located slightly below the center of the cornea, with a surrounding grayish-white infiltration zone that has well-defined edges.
Streptococcus pneumoniae keratitis often arises following trauma or chronic dacryocystitis, presenting as an oval ulcer centered in the deep layers of the corneal stroma with creeping edges. Elastic membrane folds may radiate from the ulcer margins, often accompanied by hypopyon and fibrinous deposits on the corneal endothelium, and in severe cases, corneal perforation may result.
Pseudomonas aeruginosa keratitis frequently manifests as rapidly progressing liquefactive necrosis of the cornea, often following foreign body removal or infection associated with contact lens use. The condition has an acute onset with rapid progression, presenting with intense pain, severe mixed hyperemia, and conjunctival edema. Corneal infiltration expands rapidly, accompanied by widespread liquefactive necrosis of the stroma. The ulcer surface is covered with large amounts of thick purulent discharge, often yellow-green in color, with surrounding grayish-white or yellowish-white stromal infiltration rings. Significant hypopyon is common. If the infection is uncontrolled, it can lead to corneal necrosis, perforation, prolapse of intraocular contents, or endophthalmitis.
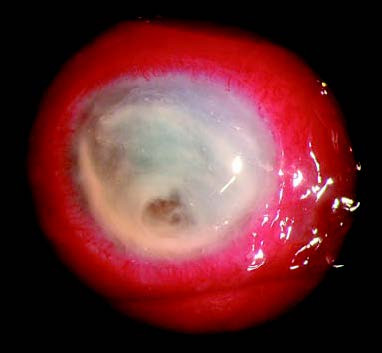
Figure 2 Pseudomonas aeruginosa corneal ulcer
Extensive liquefactive necrosis of the corneal stroma, with the ulcer surface covered by large amounts of thick purulent or mucopurulent discharge. A yellowish-white infiltration ring is visible in the stromal tissue surrounding the ulcer.
Corneal infections caused by other Gram-negative bacilli lack specific clinical signs, often accompanied by mild anterior chamber inflammatory responses. Infections caused by Klebsiella tend to occur in cases with chronic epithelial conditions, while Moraxella-associated corneal ulcers are more frequent among individuals with alcohol dependency, diabetes, or immunodeficiencies. These ulcers often present as oval-shaped lesions in the inferior cornea with a gradual infiltration into the deep stroma, clear margins, and minimal hypopyon.
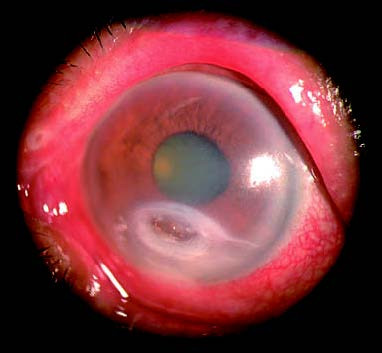
Figure 3 Moraxella corneal ulcer
An oval-shaped ulcer located in the inferior region of the cornea, gradually infiltrating into the deeper stromal layers. The lesion has clear boundaries and is accompanied by a small amount of hypopyon in the anterior chamber.
Corneal infections caused by Neisseria gonorrhoeae or Neisseria meningitidis develop rapidly and are highly aggressive, with findings such as pronounced eyelid and conjunctival edema, thick purulent discharge, and stromal corneal infiltration, necrosis, and ulceration. These infections, particularly in neonates, frequently result in corneal perforation.
Diagnosis
The clinical symptoms and signs of keratitis can lose their typical characteristics due to factors such as differences in the virulence of pathogens, the health condition of the patient's cornea, or the local use of antibiotics. The use of corticosteroids can also reduce the clinical signs of corneal inflammation. These factors often make the clinical presentation of keratitis atypical. Therefore, it is usually not possible to make an etiological diagnosis based solely on clinical manifestations. Before initiating treatment, performing a scraping from the edge of the infiltrative lesion for smear staining and bacterial examination can aid in early etiological diagnosis. A definitive etiological diagnosis requires bacterial culture along with antibiotic susceptibility testing, providing a foundation for selecting effective antibiotics.
Treatment
Bacterial keratitis can lead to rapid destruction of corneal tissue. For suspected cases, it is necessary to promptly initiate active treatment. Treatment for first-time cases can begin with broad-spectrum antibiotics based on clinical features and ulcer severity. Subsequent adjustments should be made based on bacterial culture and susceptibility testing results to ensure the use of effective antibiotics. The goal of antibiotic therapy is to quickly eradicate the pathogen. As each antibiotic has a specific antimicrobial spectrum, broad-spectrum antibiotics or a combination of two or more antimicrobial agents are necessary for initial treatment.
For infections caused by gram-positive (G+) cocci, cephalosporins are the first-line treatment, with cefazolin (50 mg/ml) being a representative drug. For G- rod-induced keratitis, aminoglycosides (e.g., 1.3%–1.5% tobramycin) are the antibiotics of choice. In cases of mixed bacterial keratitis or where Gram staining yields inconclusive results, combining cephalosporins with aminoglycosides is recommended as an initial strategy.
Fluoroquinolones exhibit strong bactericidal activity, have a broad antimicrobial spectrum, and are effective against both G- bacteria and many G+ bacteria, including drug-resistant staphylococci. Their combination with cephalosporins enhances antibacterial efficacy. The combination of cephalosporins and fluoroquinolones is considered a reasonable choice for treating vision-threatening bacterial keratitis. For keratitis caused by Streptococcus or Neisseria species, penicillin G is the preferred treatment. For penicillin-resistant Neisseria infections, ceftriaxone is often used. Vancomycin demonstrates excellent activity against both G+ cocci and rods, particularly with high sensitivity to drug-resistant Staphylococcus epidermidis and Staphylococcus aureus, and is regarded as a second-line treatment for bacterial keratitis.
The local application of antibiotics remains the most effective approach for treating bacterial keratitis. Various dosage forms are available, including eye drops, ointments, gels, and sustained-release formulations. During the acute phase, the intensified use of high-concentration antibiotic eye drops at frequent intervals helps achieve therapeutic drug levels in the corneal stroma. Ointments and gels can prolong drug retention on the ocular surface, provide lubrication, and ensure continued medication effect, making them particularly suitable for children or during nighttime.
Subconjunctival injection can increase drug concentration in the cornea and anterior chamber but may cause localized irritation. Some studies suggest that intensive application of antibiotic eye drops can achieve a therapeutic effect comparable to that of subconjunctival injections. Consequently, this approach is typically reserved for specific scenarios.
Systemic medication is generally unnecessary for this condition. However, when corneal ulcer perforation occurs or when keratitis shows signs of spreading intraocularly or systemically, systemic antibiotics are warranted in conjunction with local therapies. Treatment adjustments should be promptly made based on microbiological examination and sensitivity test results. After achieving disease control, continued local therapy for a period of time is critical to prevent recurrence of the infection, especially in cases of Pseudomonas aeruginosa-induced corneal ulcers.
For cases complicated by iridocyclitis, 1% atropine eye drops or ointments may be used for mydriasis. The local application of collagenase inhibitors, such as glutathione or cysteine, can help slow ulcer progression. In cases where medical treatment is ineffective, the ulcer fails to heal, or the disease worsens with corneal perforation either imminent or already present, surgical interventions such as conjunctival flap coverage or corneal transplantation may be considered as appropriate options.