Otosclerosis, also known as otospongiosis due to its pathological characteristics, is a condition characterized by focal bone resorption in the bony labyrinth of the inner ear, followed by vascular-rich spongy degeneration and bone proliferation. Over time, with reduced vascularity and dense bone deposition, sclerotic lesions develop. Clinically, it is marked by progressive conductive hearing loss in one or both ears, with sensorineural hearing loss potentially occurring in the later stages.
Otosclerosis typically originates as a primary lesion in the bony labyrinth, most frequently in the area anterior to the oval window. If the lesion remains confined within the bony walls of the labyrinth without causing clinical symptoms, it is known as histological otosclerosis and is usually detected only during post-mortem temporal bone histological examination. If the lesion extends beyond the bony walls, affecting the annular ligament and the stapes and subsequently limiting or fixing stapes movement, it results in progressive conductive hearing loss. This is referred to as clinical otosclerosis or stapedial otosclerosis. Furthermore, if the lesion involves the cochlear region or extends into the internal auditory canal, causing damage to the cochlea or degeneration of the auditory nerve, it leads to sensorineural hearing loss and is referred to as cochlear otosclerosis.
In 1704, Valsalva conducted a detailed post-mortem examination of a deaf patient and was the first to describe hearing loss caused by fixation of the stapes footplate to the oval window. In 1841, Toynbee identified stapes rigidity in a series of temporal bone specimens, proposing it as a common cause of hearing loss. In 1893, Politzer confirmed that this was a primary sclerotic lesion encapsulating the bony labyrinth, coining the term "otosclerosis." Siebenmann later described the key histopathological feature of otospongiosis as spongy vascular-rich bone replacing normal dense bone in the otic capsule.
The prevalence of clinical otosclerosis is as high as 0.5% among Caucasians and is about 2.5 times more common in women compared to men. The condition typically affects individuals in their middle to early adult years.
Etiology and Pathogenesis
The exact cause of otosclerosis remains unknown, as the condition is regarded as multifactorial and influenced by a combination of genetic and environmental factors. Potential contributing factors include:
Genetic Factors
Approximately 60% of clinical otosclerosis patients have a family history of the condition. Otosclerosis is considered an autosomal dominant hereditary disorder with a variable penetrance rate of 25-40%. Some researchers also view it as a multifactorial or polygenic inherited condition. The gene associated with otosclerosis has been mapped to chromosome 15q25-q26.
Developmental Factors
During the process of development and ossification, an embryonic cartilaginous fissure known as the fissula ante fenestram remains at the anterior edge of the oval window in the bony labyrinth. Under normal circumstances, this fissure is ossified and closed during childhood. In some individuals, due to defects in development or ossification, residual embryonic fibrous connective tissue bundles and cartilaginous remnants around the fissure experience abnormal bone regeneration in adulthood, leading to the development of otosclerosis.
Endocrine Dysregulation
Otosclerosis is more prevalent during puberty, and the incidence is higher in females. Hormonal changes associated with pregnancy, childbirth, and menopause can accelerate disease progression. Many female patients report initial hearing loss during their first pregnancy, with significant worsening of symptoms after delivery. This phenomenon is thought to be linked to fluctuations in estrogen levels, as estrogen can activate bone resorption and contribute to the pathogenesis of spongy bone lesions.
Immune Mechanisms
Histological and immunohistochemical studies of otosclerotic lesions have revealed chronic inflammatory reactions within the otic capsule, supporting the theory that otosclerosis is an inflammatory reactive condition. This is characterized by infiltration of macrophages, T lymphocytes, B lymphocytes, HLA-DR-positive cells, and plasma cells. Furthermore, inflammatory cell invasion of blood vessels may contribute to bone resorption.
Pathological studies have identified alterations in glycosaminoglycan synthesis, reduced collagen fibers, and collagen breakdown in active sclerotic lesions, similar to changes observed in conditions such as rheumatoid arthritis. This has led to the hypothesis that otosclerosis may involve an autoimmune component. Reports have also highlighted the presence of autoantibodies against types I, II, III, VI, IX, and XI collagens in otosclerotic lesions, suggesting that cartilage-specific autoimmune mechanisms may play a role in the disease process.
Enzyme Metabolic Dysregulation
Research has shown that enzymatic metabolic disturbances contribute to stapes fixation, with increased activity of certain proteolytic and hydrolytic enzymes implicated in the pathological mechanism of otosclerosis.
Viral Factors
Studies suggest a connection between various viruses and otosclerosis, including influenza virus, rubella virus, and paramyxoviruses (measles virus, parainfluenza virus, and mumps virus). Measles virus exposure is considered a significant risk factor for otosclerosis. Research has detected nucleic acids of the measles virus in the stapes footplate of otosclerosis patients and measles virus-specific antibodies in the perilymph fluid of the inner ear. Otosclerosis is thought to represent an inflammatory bone resorption disease of the temporal bone associated with the measles virus. Active otosclerosis cases have also exhibited evidence of rubella virus. Furthermore, structures resembling paramyxovirus nucleocapsids have been observed in osteoblasts within otosclerotic tissues under electron microscopy.
Pathology
The otic capsule of the bony labyrinth consists of the outer periosteal layer, the cartilaginous endochondral layer, and the endosteal layer. The pathological hallmark of otosclerosis is abnormal bone resorption and sclerosis within the otic capsule and auditory ossicles.
Focal Bone Resorption and Destruction (Dissolution Phase)
The activity of osteoclasts increases, leading to bone resorption around blood vessels and the formation of fibrous spaces. Focal bone destruction and resorption occur repeatedly.
Formation of Spongy Bone Tissue (Osteogenesis Phase)
Osteoblasts in the fibrous spaces generate immature bone, and the marrow spaces enlarge to form spongy new bone.
Bone Deposition and Sclerosis (Remodeling Phase)
Recurrent bone resorption and new bone formation result in bone deposition, producing dense, sclerotic new bone.
These pathological changes can coexist or alternate in otosclerosis. Lesions typically originate in the cartilaginous endochondral layer, with 70–90% occurring at the fissula ante fenestram, subsequently involving the annular ligament and stapes footplate, leading to conductive hearing loss. Approximately 40% of cases include localized lesions in the round window or cochlear canal. Based on the balance of osteoclast and osteoblast numbers, the extent of spongy vascular cavity enlargement or narrowing, and the transition of basophilic bone to eosinophilic bone, the histopathological changes in otosclerosis have been categorized into four types: active, intermediate, resting, and mixed types.
Classification
Otosclerosis is classified by its location into stapedial otosclerosis and cochlear or labyrinthine otosclerosis:
Stapedial Otosclerosis
This form has a focal onset with slow progression, primarily affecting the vestibular window niche, annular ligament, and stapes. Limited or fixed stapes mobility leads to conductive hearing loss. This is the most common clinical form of otosclerosis.
Cochlear or Labyrinthine Otosclerosis
Lesions occur in the round window, cochlear canal, semicircular canals, and the bony wall of the internal auditory canal. These lesions may extend to the endosteal layer, interfering with the movement of the basilar membrane and inner ear microcirculation. Additionally, toxic enzymes (e.g., cytotoxic enzymes) from the lesion can be released into the perilymph, causing damage to hair cells and the stria vascularis, resulting in vertigo and sensorineural hearing loss.
Since lesions can occur in multiple areas, stapedial otosclerosis and cochlear/labyrinthine otosclerosis may coexist.
Symptoms and Signs
The primary clinical manifestations include bilateral, progressive hearing loss and low-pitched tinnitus, occurring without any evident triggers. Symptoms are generally limited to the ear and do not include aural fullness or otorrhea.
Progressive Hearing Loss
Patients experience slow, progressive, and insidious onset of conductive or mixed hearing loss in one or both ears, with the degree of loss potentially differing between sides. Unilateral cases are less common. Early in the disease, patients are often unaware of the hearing loss, which may take several years or even more than a decade to significantly impact daily life and work.
Tinnitus
Most patients present with a persistent, low-pitched tinnitus, often described as a "buzzing" sound. Pulsatile low-pitched tinnitus is thought to result from vascular proliferation within the lesion. High-pitched tinnitus is less common and may suggest cochlear involvement.
Vertigo
Some patients may experience mild and transient vertigo, typically occurring when head movement suddenly stops. In rare cases, vertigo may last for several hours or more, which has been hypothesized to result from elevated inner ear pressure due to coexisting otosclerosis and endolymphatic hydrops. Symptoms of vertigo often resolve after surgery.
Autophony
Patients often report hearing their own voice distinctly and softly and describe their speech as articulate.
Willis' Paracusis
Affected individuals may notice improved hearing of speech in noisy environments compared to quiet settings, as ambient noise masks background sounds and the speaker tends to raise their voice in noisy conditions. This phenomenon, whereby conductive hearing loss blocks background noise and falsely enhances the perception of speech, is termed Willis' paracusis.
Higher Prevalence in Women
The condition is more common in women, with disease progression often accelerating during pregnancy and postpartum periods. Hormonal changes during these stages are thought to contribute to this acceleration.
Examinations
Otoscopy
Most patients present with a relatively wide ear canal. The tympanic membrane remains intact with a normal shine. In some cases, a faint reddish area can be observed in the posterosuperior quadrant, which is a sign of congestion in an active lesion within the promontory region. This is referred to as the Schwartze sign.
Hearing Function Tests
Tuning Fork Tests
Weber Test
The sound lateralizes to the side with more severe conductive hearing loss.
Rinne Test
Negative results indicate bone conduction (BC) is greater than air conduction (AC).
Schwabach Test
Bone conduction is prolonged.
Gelle Test
The result is negative. In cases of stapes fixation, changes in external ear canal air pressure do not affect the intensity of bone-conducted tuning fork sounds. It should be noted that poor tympanic membrane mobility (due to calcification, thickening, or adhesion), fixation of the incus or stapes, or disruption of the ossicular chain can all prevent external ear canal pressure changes from being transmitted to a mobile stapes, possibly leading to false negatives. Therefore, the Gelle Test is not considered a reliable method to differentiate stapes fixation.
A combination of negative Rinne Test, Weber Test lateralizing to the side with severe conductive hearing loss, and prolonged bone conduction on Schwabach Test is referred to as the Bezold triad.
Pure-Tone Audiometry (PTA)
Findings usually indicate either purely conductive hearing loss or mixed hearing loss of varying degrees if the cochlea is involved.
Early Stage
Bone conduction remains normal, while air conduction displays a rising pattern typical of conductive hearing loss, with an air-bone gap of 30–45 dB.
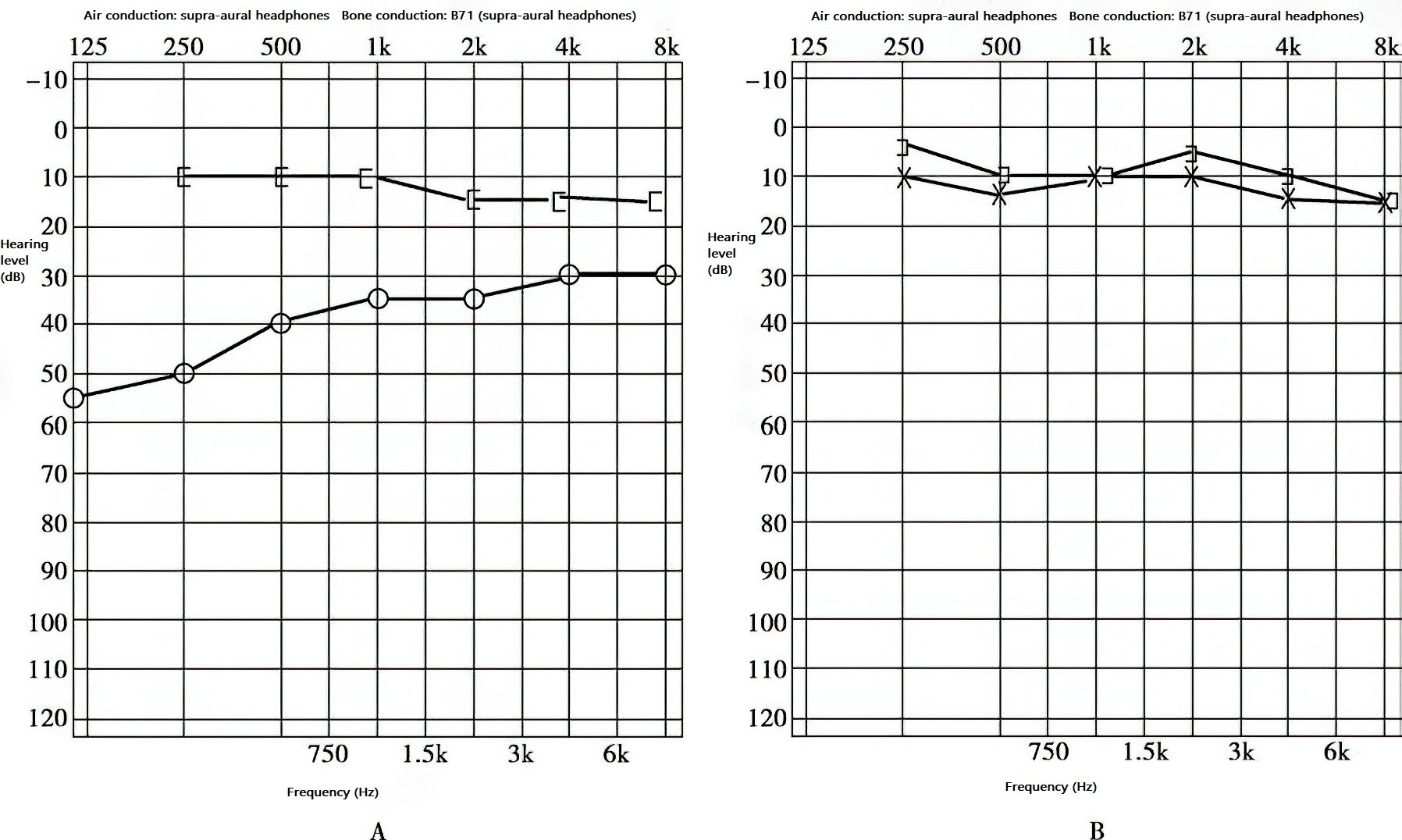
Figure 1 Early-stage hearing in right-sided otosclerosis
A. Conductive hearing loss.
B. Normal hearing in the left ear.
Intermediate Stage
Impedance mismatch caused by stapes fixation leads to a notable dip in the bone conduction curve at 2 kHz, known as the Carhart notch. Air conduction typically shows a flat pattern, while the air-bone gap exceeds 45 dB.
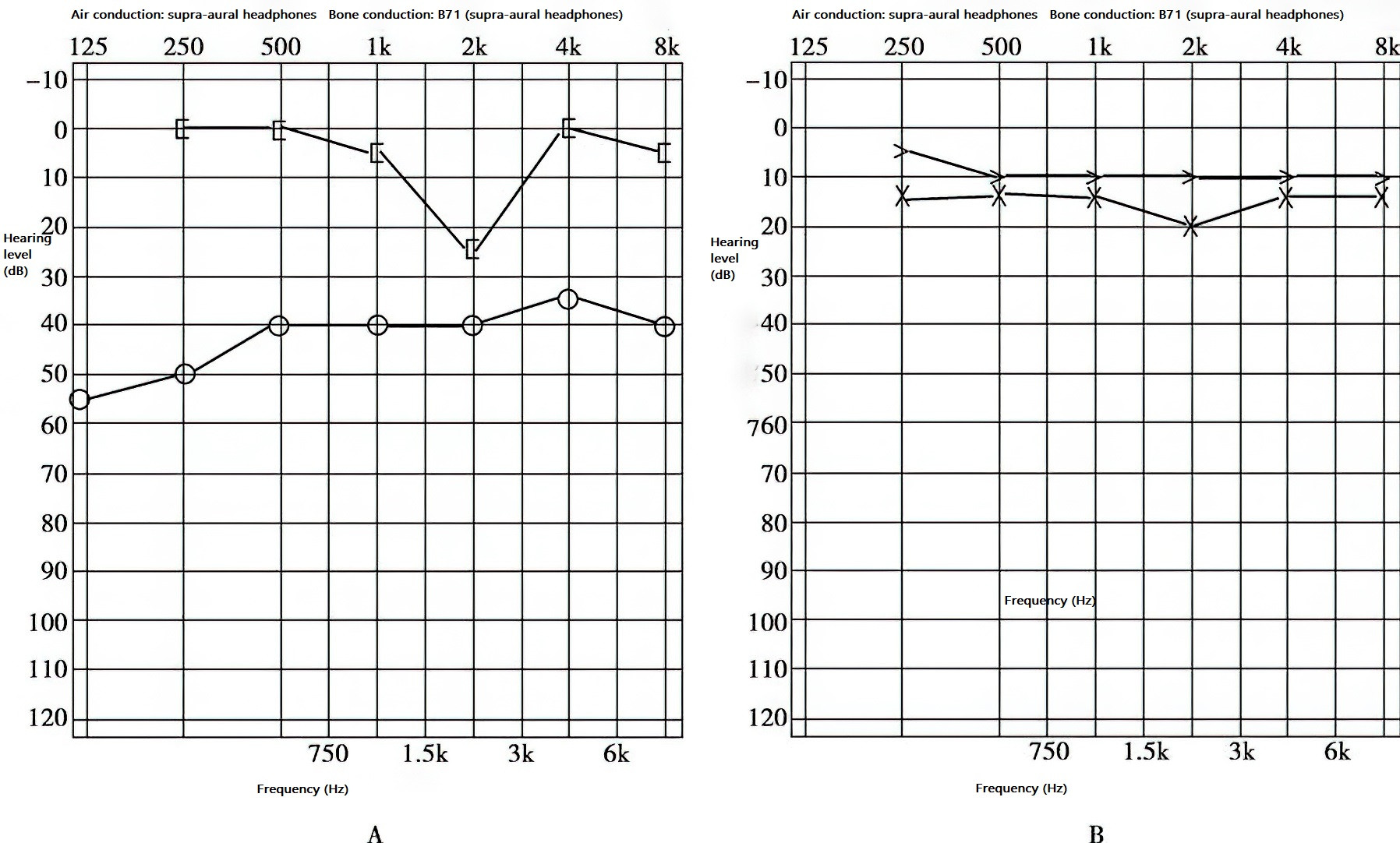
Figure 2 Mid-stage hearing in right-sided otosclerosis
A. Conductive hearing loss with Carhart notch.
B. Normal hearing in the left ear.
Late Stage
Both air conduction and bone conduction curves show downward trends. While a significant air-bone gap may still persist in the low-frequency range, at frequencies above 1 kHz, the air-bone gap may diminish, with both curves showing a downward-sloping configuration.
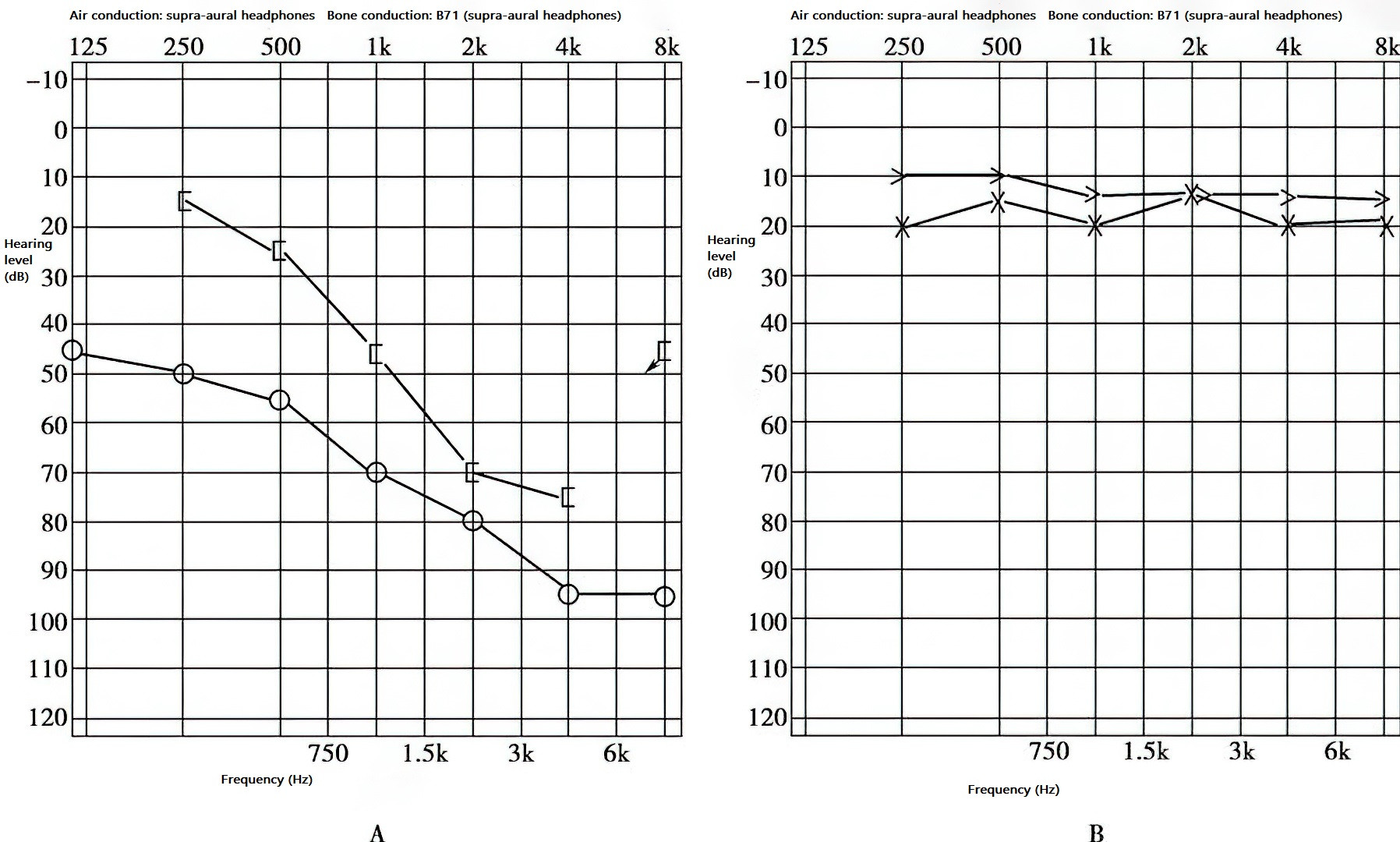
Figure 3 Late-stage hearing in right-sided otosclerosis
A. Mixed hearing loss.
B. Normal hearing in the left ear.
Tympanometry
Tympanogram
Early-stage otosclerosis shows a type A tympanogram. As stapes fixation progresses, tympanic membrane mobility becomes restricted, resulting in a low-compliance type As curve.
Static Compliance
This remains within normal limits.
Stapedius Reflex
In the early stages of otosclerosis, stapes rigidity causes reflex thresholds to elevate, or a "biphasic on-off type" double curve may appear. In later stages, when the stapes becomes fixed, stapedius reflexes are absent.
Eustachian Tube Function
Normal tympanometric pressure curves are observed.
Imaging Studies
High-resolution thin-slice spiral CT scanning typically reveals normal mastoid cells as well as well-developed ossicles and inner ear structures without malformations. Some cases allow precise localization of otosclerotic lesions. Observations may include localized thickening of the stapes footplate, abnormal changes in the oval window, round window, or semicircular canals, and heterogeneous bone density in the labyrinth.
Stapedial otosclerosis on CT shows abnormal density around the oval window and/or round window with either widening or narrowing of the niches. Thickening of the stapes footplate may also be observed.
Cochlear otosclerosis on CT displays a ring-like area of low-density surrounding the cochlea, with the classic "double ring sign" seen in typical cases.
Grading
Stapedial Otosclerosis Grading
Grade 1
Otospongiotic changes are confined to the fissula ante fenestram.
Grade 2
Otospongiotic changes extend to the anterior half of the vestibular window and crura.
Grade 3
Otospongiotic changes involve the entire vestibular window niche.
High-resolution thin-slice spiral CT scanning often shows normal mastoid air cells and well-developed ossicles and middle ear structures. Studies have reported that approximately 74% of otosclerosis cases can be localized to the lesion site using high-resolution CT (HRCT) with 0.5 mm slice thickness. Findings may include focal thickening of the stapes footplate and abnormal changes at the vestibular window, round window, semicircular canals, or labyrinth bone density, with narrowing or widening of the window niche.
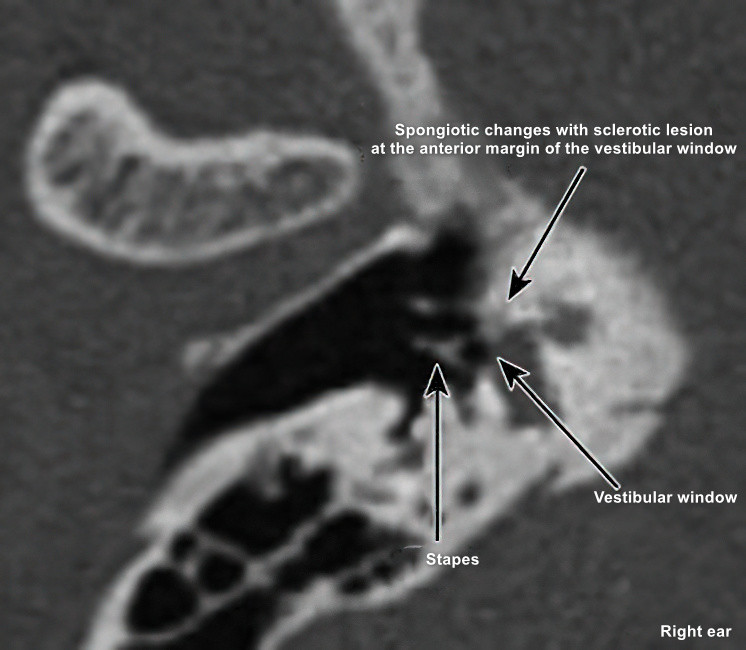
Figure 4 Sclerotic lesion involving the anterior margin of the vestibular window at the stapes
Cochlear Otosclerosis Grading
Grade 1
The range of otospongiotic changes does not exceed the diameter of one cochlear turn.
Grade 2
Otospongiotic changes involve more than one cochlear turn but do not extend to the entire otic capsule.
Grade 3
Otospongiotic changes involve the entire otic capsule.
Cochlear otosclerosis is associated with the presence of the "double ring sign" on imaging.
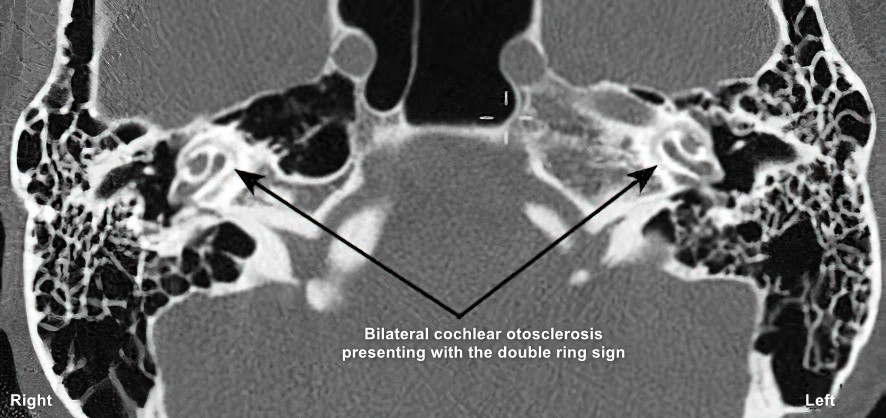
Figure 5 Inhomogeneous bone density in the cochlea, formation of "double ring sign"
Diagnosis
A diagnosis of stapedial otosclerosis can be established when patients present with bilateral progressive conductive hearing loss without any apparent trigger, potentially accompanied by low-frequency tinnitus, intact tympanic membranes, or the presence of Schwartze sign. Eustachian tube function remains normal. Characteristic findings on tuning fork tests include the Bezold triad and a negative Gelle test. Audiological examinations typically show conductive hearing loss, with a prominent air-bone gap in low frequencies and the bone conduction curve occasionally exhibiting a Carhart notch. Tympanometry results often reveal a type A or As curve. These typical features strongly support the diagnosis of stapedial otosclerosis.
When patients present with bilateral progressive sensorineural hearing loss disproportionate to their age, with intact tympanic membranes and potentially a Schwartze sign, pure-tone audiometry often shows reductions in both air and bone conduction thresholds. The low-frequency air-bone gap typically measures 15–20 dB. Tympanometric findings are usually type A or As. If the patient has a family history of otosclerosis, cochlear otosclerosis or late-stage otosclerosis should be considered. Imaging studies may reveal inhomogeneous bone density in the bony labyrinth or inner auditory canal walls, or bone deformities, supporting the diagnosis of labyrinthine otosclerosis.
In some cases, HRCT imaging can localize the lesions associated with otosclerosis.
Differential Diagnosis
Differentiation from other potential causes of conductive hearing loss is necessary, including congenital ossicular chain malformations, Van der Hoeve syndrome, and other conditions such as secretory otitis media, adhesive otitis media, tympanic effusion, tympanic membrane adhesions, intact tympanic membrane tympanosclerosis, acquired primary middle ear cholesteatoma, or Paget’s disease (osteitis deformans).
Distinction between labyrinthine otosclerosis and other conditions causing progressive sensorineural hearing loss must also be made. These include delayed-onset hereditary sensorineural hearing loss, chronic ototoxicity, and systemic factors such as diabetes-related sensorineural hearing loss.
Treatment
Surgical intervention is the main treatment for all stages of stapedial otosclerosis. Early and intermediate stages tend to show favorable outcomes, while late-stage results are often less satisfactory. For labyrinthine otosclerosis, in addition to fitting hearing aids, sodium fluoride may be considered.
Observation or Conservative Management
When early-stage hearing loss does not significantly affect daily life or work, regular follow-up is recommended without surgery.
For patients with contraindications to surgery or those who decline surgery, hearing aids can be considered.
Labyrinthine otosclerosis often involves sensorineural hearing loss, for which hearing aids are advised.
The efficacy of pharmacological treatment remains uncertain. Sodium fluoride can be trialed orally, and the therapeutic effect should be assessed after two years. Approximately 50% of patients achieve disease stability, while 30% experience progression. This treatment is not routinely recommended and is reserved for cases of uncontrolled cochlear otosclerosis. The clinical utility of vitamin D and calcium supplementation remains controversial.
Surgical Treatment
In 1953, Rosen discovered during preparations for fenestration surgery that stapes mobilization could restore hearing in patients. However, due to the possibility of stapes refixation and the difficulty of mobilizing some stapes, Shea performed the first stapedectomy for otosclerosis in 1956. This involved removing the stapes in a female otosclerosis patient and implanting a prosthesis attached to the oval window covered with a vein graft, achieving significant results.
Since 1962, Shea and Marquet developed a less invasive technique to reduce inner ear damage, creating a small opening in the center of the stapes footplate to accommodate a piston prosthesis. This marked the advent of the "stapedotomy" era, a technique now widely adopted worldwide.
In 1980, Perkins first used the argon laser during stapes surgery. Stapes prostheses are now made of biocompatible materials such as titanium, Teflon, gold, or platinum. Titanium alloy prostheses are widely used in clinical practice for their favorable properties.
In clinical practice, patients with otosclerosis who experience significant hearing loss—air conduction hearing loss exceeding 45 dB or an air-bone gap greater than 20 dB—that affects their daily communication or work may be considered for surgery. Prior to surgery, thorough discussions with the patient about the risks, complications, and alternative options are essential. Less than 1% of surgical cases may result in complete hearing loss in the operative ear. Although the risk of sensorineural hearing loss due to surgery is very low, this possibility should still be disclosed. For patients over the age of 55, the risk of surgery-induced sensorineural hearing loss increases and must be specifically communicated.
The common surgical techniques available include stapes mobilization and various types of stapedectomy surgeries:
Stapes Mobilization (Stapediolysis)
Techniques include indirect and direct mobilization. The incudostapedial joint and stapes head are exposed, and mobilization techniques are used to loosen the stapes footplate. However, this procedure requires careful handling as there is a risk of incudostapedial joint dislocation or perilymphatic fistula. Due to its associated risks, this surgical approach has been largely phased out.
Stapedectomy
This procedure encompasses several variations, including total removal of the footplate, fragmented removal in sections, partial removal of the footplate, or perforation of the footplate. Since stapedectomy poses a higher risk of trauma, it has predominantly been replaced by the stapedotomy (small window) technique.
Stapedotomy (Small Window Technique)
This approach involves creating an opening in the stapes footplate using tools such as hand drills, power drills, or lasers. Stapedotomy is considered safe and minimally invasive, with milder postoperative reactions, and is the most widely used surgical method.
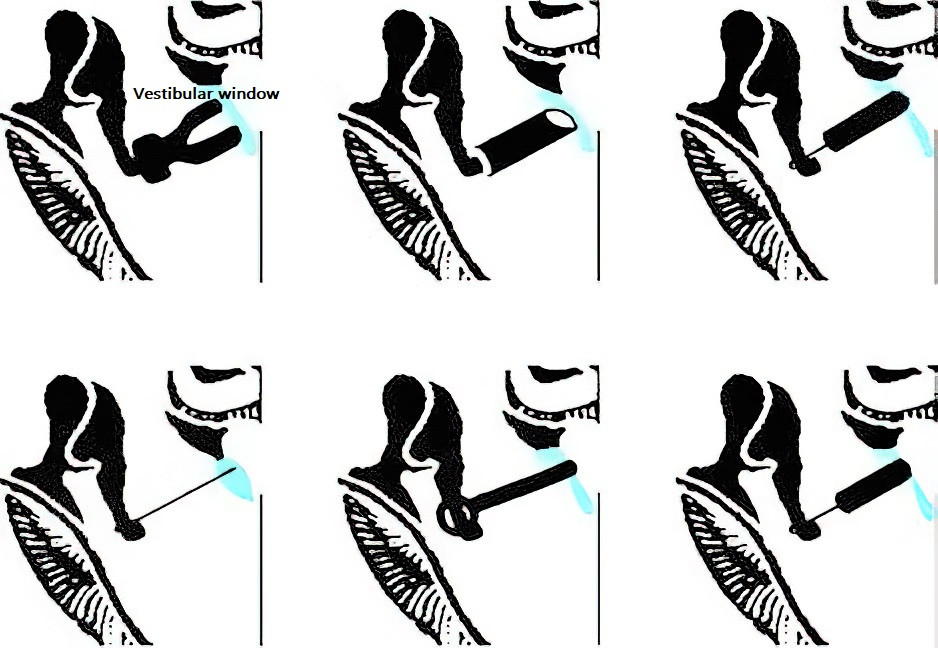
Figure 6 Schematic diagram of various types of artificial stapes implantation
Several types of artificial stapes prostheses have been used in clinical practice, including:
- Polyethylene columella prostheses.
- Stainless steel prostheses with fat plugs.
- Bone prostheses.
- Piston prostheses made of polytetrafluoroethylene (PTFE) or inert light metals, such as titanium or tantalum.
- Re-implanted stapes crura.
- Titanium alloy prostheses.
Titanium piston prostheses are now the most frequently used implants in clinical settings.
The surgical procedure includes the following steps:
- Partial removal or drilling of the lateral wall of the attic to fully expose the stapes and the horizontal segment of the facial nerve.
- Measurement of the distance between the long process of the incus and the stapes footplate to select an appropriately sized prosthesis.
- Creation of a small opening in the stapes footplate.
- Implantation of the prosthesis into the vestibule and secure attachment to the long process of the incus using specialized instruments.
- Separation of the incudostapedial joint.
- Cutting or fracturing the stapes crura, removal of the structures above the stapes footplate, and verification of the prosthesis’s mobility.
In modern surgical approaches, the stapedotomy technique is used for reconstructing sound transmission. Prosthesis pistons typically have diameters ranging from 0.4 to 0.6 mm, with the diameter of the stapedotomy opening slightly larger by 0.2 mm. The prosthesis ideally penetrates approximately 0.5 mm into the vestibule but must not exceed 1 mm. Excess length can damage the saccule and cause vertigo or sensorineural hearing loss, while insufficient length increases the risk of dislocation. The opening in the stapes footplate is typically located in the posterior-central area, the point farthest from the saccule.
If the prosthesis is attached to the incus, the procedure is referred to as incus-stapedotomy, while attachment to the malleus handle is termed malleo-stapedotomy.
Fenestration of the Inner Ear
Also known as semicircular canal fenestration, this technique has been abandoned due to its limited efficacy and the advancement of bone conduction hearing devices like BAHA and bone-anchored implants.
Surgical Intervention for Advanced Severe Sensorineural Hearing Loss in Otosclerosis
Although various methods have been explored for treating advanced otosclerosis, no standardized guidelines have been established.
Major treatment options include stapes surgery combined with hearing aids or cochlear implantation.
The most serious complication of stapes surgery is sensorineural hearing loss, most prominently at high frequencies. Additional complications include incus loosening and postoperative vertigo. Surgical management of patients with mixed hearing loss requires even greater caution. The key to preventing complications lies not only in the surgeon’s expertise with middle ear microsurgery but also in minimizing vestibular stimulation during the procedure. Recent advancements—such as optimized prosthetic implants, refinement of the stapedotomy technique, and the use of laser perforation—have contributed to significant improvements in reducing postoperative complications like vertigo and tinnitus, although improvements in hearing outcomes remain largely unchanged.
Prognosis
Otosclerosis progresses slowly and initially presents as conductive hearing loss. Over time, this may develop into mixed or sensorineural hearing loss. Currently, no medications can effectively treat or halt the progression of the disease. Surgery can improve hearing, but it cannot prevent further lesion development. Rapidly progressing cases with multiple foci may lead to severe sensorineural hearing loss.