Congestive heart failure refers to a pathological state in which the heart's functional capacity (either systolic or diastolic) declines, resulting in an absolute or relative insufficiency in cardiac output to meet the metabolic demands of the body's tissues. This is accompanied by disturbances in neuroendocrine regulation and represents one of the critical conditions in pediatrics.
Etiology
Heart failure in children occurs most commonly within the first year of life, with congenital heart diseases being the leading cause. In congenital heart disease, both increased afterload caused by outflow tract obstruction and increased preload caused by left-to-right shunting or valvular regurgitation can lead to heart failure. Other causes include conditions such as viral myocarditis, Kawasaki disease, rheumatic heart disease, cardiomyopathy, and endocardial fibroelastosis. Other common triggers of heart failure in children include anemia, malnutrition, electrolyte imbalances, and severe infections.
Pathophysiology
The progression from normal cardiac function to heart failure typically involves a compensatory phase during which the heart undergoes myocardial hypertrophy, enlargement, and an increase in heart rate. The elongation and thickening of myocardial fibers initially enhance contractility and increase cardiac output. If the underlying cause persists, these compensatory mechanisms progressively evolve, leading to increased myocardial energy consumption, relative coronary insufficiency, reduced myocardial contraction speed, and weakened contractile force. When heart rate becomes excessively rapid, diastolic duration is shortened, leading to reduced cardiac output. Heart failure manifests when the compensatory mechanisms no longer suffice to meet the body's metabolic demands.
When cardiac output falls below the normal resting level, the condition is classified as low-output heart failure. In contrast, heart failure resulting from conditions such as hyperthyroidism, severe anemia, or arteriovenous fistulas leads to increased systemic circulation volume, elevated venous return, and cardiac output that, while still reduced after heart failure develops, remains higher than the normal resting level. This is referred to as high-output heart failure.
In heart failure, reduced ventricular ejection during contraction leads to an increase in residual blood volume within the ventricles, elevated diastolic filling pressure, and concurrent tissue hypoxia along with venous and atrial congestion. Tissue hypoxia activates the sympathetic nervous system, causing vasoconstriction in the skin and visceral organs to redistribute blood flow and ensure perfusion of essential organs. Renal vasoconstriction reduces renal blood flow and glomerular filtration rate, increasing the secretion of renin and subsequently aldosterone. This enhances sodium reabsorption in the proximal and distal renal tubules, leading to sodium and water retention, increased blood volume, and fluid accumulation in the interstitial spaces.
In recent years, the role of neuroendocrine regulation in the progression of heart failure has been increasingly recognized. The reduction in cardiac output activates the sympathetic nervous system and the renin-angiotensin-aldosterone system, disrupting the regulation of the β-receptor-adenylate cyclase system. This results in peripheral vasoconstriction and further sodium and water retention, exacerbating ventricular remodeling and the progression of heart failure.
Ventricular overload can be categorized into volume overload and pressure overload. The compensatory capacity of the myocardium in cases of mild or moderate volume overload is generally better than in pressure overload. For example, atrial septal defects may involve significant shunting but typically represent diastolic overload, rarely leading to heart failure during childhood. Pulmonary valve stenosis represents systolic overload, often resulting in earlier onset of heart failure. Coarctation of the aorta accompanied by a patent ductus arteriosus involves both systolic and diastolic overload, which can lead to fatal outcomes during the neonatal period.
Clinical Manifestations
In older children, the symptoms of heart failure resemble those seen in adults and primarily include fatigue, lack of appetite, shortness of breath after exertion, and coughing. At rest, tachycardia, shallow and rapid breathing, jugular vein distention, hepatomegaly with tenderness, and a positive hepatojugular reflux test may be observed. Severe cases may present with orthopnea, pulmonary crackles at the lung bases, peripheral edema, and markedly reduced urine output. Cardiac auscultation often reveals reduced first heart sound intensity at the apex and the presence of a gallop rhythm, in addition to murmurs or abnormal heart sounds caused by the underlying cardiac condition.
The clinical features of heart failure in infants and young children have distinctive characteristics. Common symptoms include shallow, rapid breathing with rates ranging from 50 to 100 breaths per minute, feeding difficulties, poor weight gain, irritability, excessive sweating, and weak crying. Pulmonary findings may include dry rales or wheezing. Edema typically begins in the face and eyelids and, in severe cases, cyanosis may be observed in the nasolabial triangle.
Diagnosis
Clinical Diagnostic Criteria
These include:
- Increased heart rate at rest, exceeding 180 beats per minute in infants or 160 beats per minute in young children, which cannot be attributed to fever or hypoxia.
- Respiratory distress and a sudden increase in cyanosis, with resting respiratory rate exceeding 60 breaths per minute.
- Hepatomegaly, with the liver palpable more than 3 cm below the costal margin, or a noticeable increase in liver size over a short period under close observation, not explained by conditions such as diaphragmatic displacement.
- Notable muffled heart sounds or the presence of a gallop rhythm.
- Sudden irritability, pallor, or grayish skin color, without an explanation based on the preexisting illness.
- Reduced urine output and lower limb edema, after ruling out causes such as malnutrition, nephritis, or vitamin B1 deficiency.
Additional Examinations
The first four criteria above serve as the main basis for clinical diagnosis. Additional supporting evidence can be derived from other clinical observations and the following investigations.
- N-terminal pro-B-type natriuretic peptide (NT-proBNP): An important biomarker for heart failure, useful for diagnosis, differential diagnosis, evaluating severity, monitoring treatment efficacy, and assessing prognosis.
- Chest X-ray: Typically shows generalized cardiac enlargement, reduced pulsation, increased pulmonary markings, increased hilar or perihilar shadows, and pulmonary congestion.
- Electrocardiogram (ECG): Although not indicative of the presence of heart failure, it may aid in identifying the underlying cause and guide the use of digitalis.
- Echocardiography: May reveal enlargement of the ventricular and atrial chambers. M-mode echocardiography often demonstrates prolonged ventricular systole and a decreased ejection fraction. An E/A ratio (early diastolic filling velocity to late diastolic filling velocity) of <1 frequently suggests left ventricular diastolic dysfunction.
Treatment
General Measures
Reducing Cardiac Workload
Adequate rest, maintaining a supine or semi-recumbent position, and minimizing agitation or crying in young children may alleviate the cardiac burden. Sedatives like phenobarbital or morphine (0.05 mg/kg via subcutaneous or intramuscular injection) can often produce satisfactory results, though respiratory depression should be monitored. Limiting fluid intake as appropriate, along with providing easily digestible and nutrient-rich food, aids in reducing stress on the heart. Sodium intake in the diet should be reduced, but strict sodium restriction is seldom required.
Oxygen Therapy
This helps alleviate tissue hypoxia.
Correcting Water, Electrolyte, and Acid-Base Imbalances
The propensity for sodium and water retention, acidosis, hypoglycemia, and hypocalcemia during heart failure is especially pronounced in neonates and requires timely correction.
Digitalis Drugs
Digitalis remains one of the most widely used inotropic agents in pediatric clinical practice. It acts on the Na+-K+ ATPase enzyme in myocardial cells, inhibiting its activity. This increases intracellular sodium concentration and promotes calcium influx via the Na+-Ca2+ exchange, enhancing myocardial contractility. End-diastolic ventricular pressure is significantly reduced, alleviating symptoms of venous congestion. Digitalis also directly inhibits excessive neuroendocrine activity, particularly that of the sympathetic nervous system, and exerts negative chronotropic and dromotropic effects. Digitalis is effective in congestive heart failure caused by conditions such as left heart valve regurgitation, endocardial fibroelastosis, dilated cardiomyopathy, and certain congenital heart diseases. It is particularly beneficial in cases with tachycardia, atrial flutter, or atrial fibrillation, but shows limited efficacy when heart failure results from anemia or myocarditis.
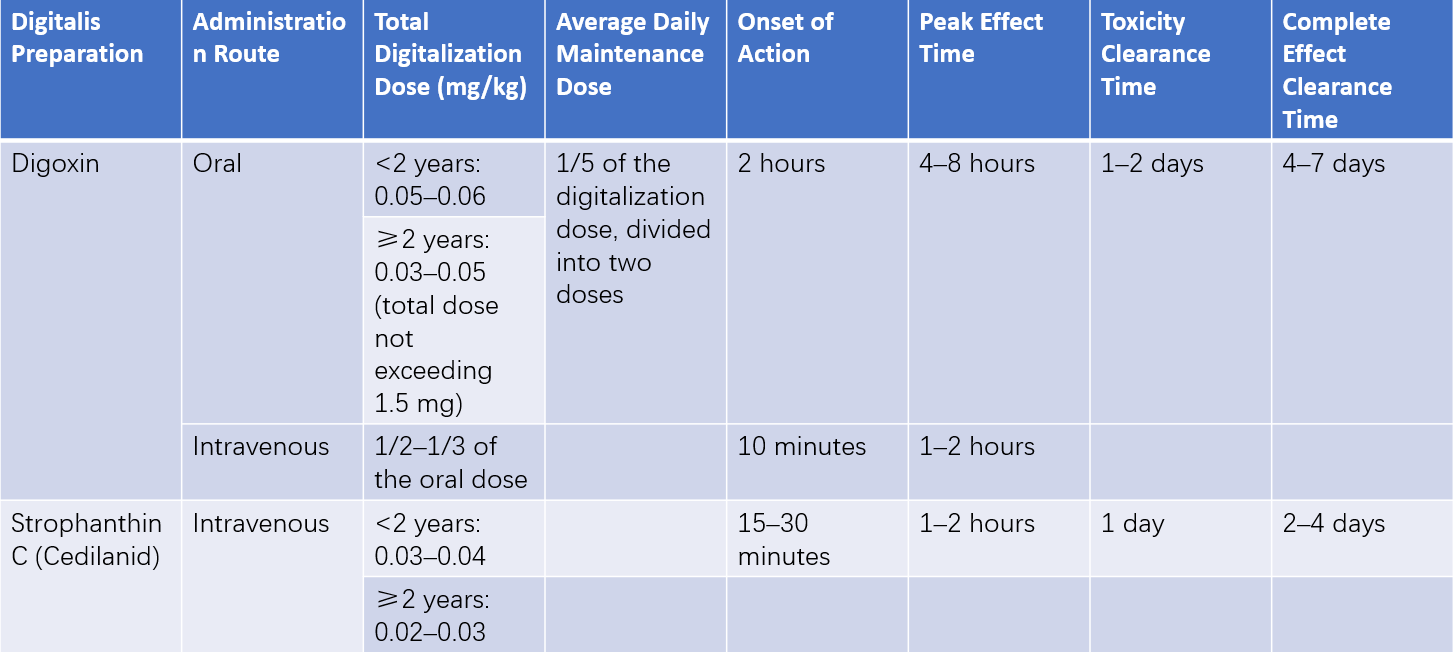
Table 1 Clinical applications of digitalis drugs
The most commonly used digitalis preparation in pediatric patients is digoxin, which can be administered orally or intravenously. Its relatively rapid onset and clearance make dosage adjustments easier, as well as the management of potential drug toxicity. The oral elixir form of digoxin has higher absorption rates. Premature infants are more sensitive to digitalis compared to full-term neonates, who in turn are more sensitive than infants. Therapeutic concentrations range from 2–4 ng/mL in infants to 1–2 ng/mL in older children. Digoxin treatment requires individualized dosing.
Digitalization
In severe cases or when oral administration is not feasible, digitalization can be achieved using either strophanthin K or intravenous digoxin. For the initial dose, half of the total digitalization dose is given first, with the remainder divided into two doses administered at 4–6-hour intervals, typically achieving digitalization within 8–12 hours in most patients. For those able to take oral medication, oral digoxin is preferred. One-third to half of the total digitalization dose is given initially, with the remainder divided into two doses administered 6–8 hours apart. For chronic heart failure, starting directly with maintenance doses of oral digoxin and allowing blood concentrations to stabilize over 5–7 days achieves similar effectiveness with lower toxicity risk compared to using loading doses.
Maintenance Doses
Maintenance doses may begin 12 hours after digitalization, typically amounting to 1/8–1/10 of the loading dose, administered twice daily at 12-hour intervals. The duration of maintenance therapy depends on the underlying condition. Long-term cases with persistent causes, such as endocardial fibroelastosis or rheumatic valvular disease, require careful dose adjustments to account for weight changes in growing children to maintain effective digoxin serum concentrations.
Considerations for the Use of Digitalis
A review of the child's digitalis usage over the previous 2–3 weeks is recommended to prevent toxicity from drug accumulation. Children with myocarditis caused by various etiologies often exhibit poor tolerance to digitalis, with doses typically reduced by one-third of the standard amount, and the saturation period kept slower than usual. Premature infants and neonates under two weeks old are at increased risk of toxicity due to immature liver and kidney function, necessitating reduced doses (about one-third to one-half of the infant dosage). Calcium supplements may potentiate the effects of digitalis and are avoided during its use. Additionally, hypokalemia increases the risk of digitalis toxicity, which requires careful monitoring.
Digitalis Toxicity
In severe heart failure or cases of poor heart function, the therapeutic dose and toxic dose of digitalis are close, which predisposes to toxicity. Children with liver or kidney dysfunction, electrolyte imbalances such as hypokalemia or hypercalcemia, myocarditis, or recent high-dose diuretic therapy are particularly susceptible to digitalis toxicity. Arrhythmias, such as atrioventricular conduction block, premature ventricular contractions, and paroxysmal tachycardia, are the most common symptoms of digitalis toxicity in children. Gastrointestinal symptoms, including nausea and vomiting, are also frequently observed, while neurological symptoms such as drowsiness, dizziness, or color vision disturbances are less common.
In the event of digitalis toxicity, discontinuation of digitalis and diuretics should occur immediately, along with potassium supplementation. Low doses of potassium salts can control premature ventricular contractions and paroxysmal tachycardia induced by digitalis. For mild cases, oral potassium chloride at 0.075–0.1 g/kg per day in divided doses is appropriate. In severe cases, intravenous infusion of 0.03–0.04 g/kg per hour may be administered, with a total dose not exceeding 0.15 g/kg and diluted in 10% glucose to a final concentration of 0.3%. Intravenous potassium is contraindicated in patients with renal impairment or those with atrioventricular conduction block.
Diuretics
Sodium and water retention represent a significant pathophysiological aspect of heart failure, making diuretics an essential component of treatment. When heart failure is not fully controlled with digitalis, or when significant edema is present, diuretics are often added. For acute heart failure or pulmonary edema, rapidly acting and potent diuretics such as furosemide or ethacrynic acid may be used, which promote sodium excretion while causing relatively less potassium loss. For chronic heart failure, a combination of thiazide diuretics with potassium-sparing diuretics is preferred, often using an intermittent dosing regimen to prevent electrolyte imbalance.
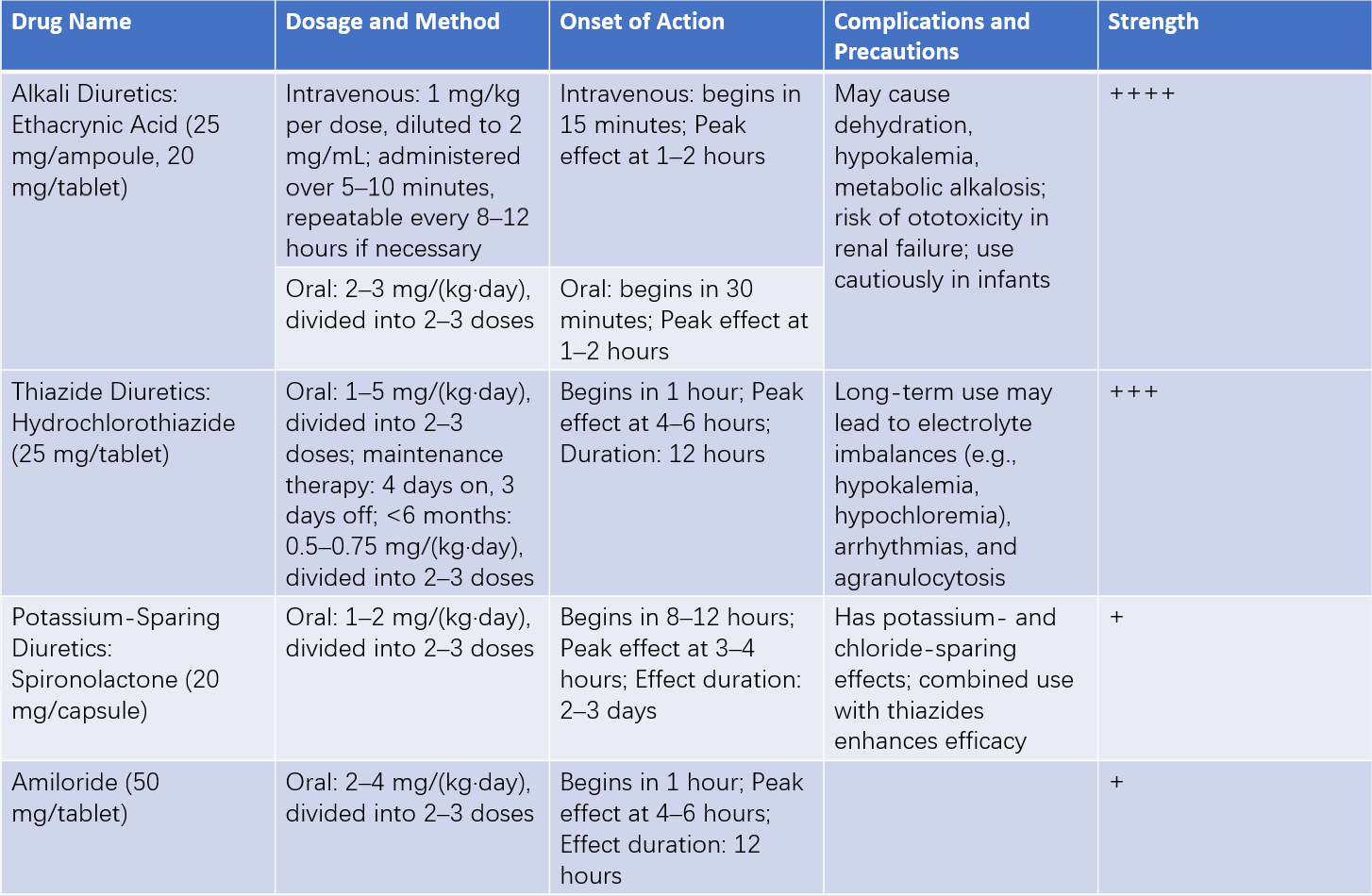
Table 2 Clinical applications of diuretics
Vasodilators
The use of vasodilators in the treatment of refractory heart failure has shown promising results. Dilation of small arteries reduces afterload, potentially increasing cardiac output, while venous dilation reduces preload and ventricular filling pressure, alleviating symptoms of pulmonary congestion. These therapies are particularly suitable for patients with elevated left ventricular diastolic pressure.
Angiotensin-Converting Enzyme (ACE) Inhibitors
These reduce the concentration of angiotensin II in circulation, effectively alleviating heart failure symptoms, improving left ventricular systolic function, preventing myocardial remodeling, reversing ventricular hypertrophy, and lowering mortality rates in heart failure patients.
Sodium Nitroprusside
This releases nitric oxide, which increases cGMP levels and relaxes vascular smooth muscle, dilating both small arteries and veins. Sodium nitroprusside has potent, rapid, but short-lived effects, particularly in acute heart failure (such as acute left heart failure or pulmonary edema) with significantly increased peripheral vascular resistance. Improved outcomes have been observed in conjunction with dopamine in postoperative low cardiac output syndrome after cardiopulmonary bypass surgery. Arterial pressure monitoring is necessary during treatment.
Phentolamine
This is an alpha-receptor blocker primarily dilating small arteries with additional venodilation effects.
Other Medications
Dopamine may be used in heart failure accompanied by hypotension, where it helps increase cardiac output and blood pressure without significantly increasing heart rate.
Heart Transplantation
For children with end-stage heart failure, heart transplantation represents a viable surgical option.
Etiological Treatment
Etiological treatment is essential, as surgical correction remains the definitive approach to resolving heart failure in congenital heart disease patients. For cases where heart failure results from hyperthyroidism, severe anemia, vitamin B1 deficiency, viral myocarditis, or toxic myocarditis, the underlying condition requires prompt and specific treatment.
Prognosis
The prognosis for heart failure depends on the severity of the condition and the adequacy of treatment. Inpatient mortality rates for heart failure range from 6% to 15%. Among children undergoing heart transplantation, infants experience a higher risk of death within the first postoperative year compared to older children. However, long-term outcomes for infants who survive the first year are generally more favorable than those for adolescents. Proactively treating the primary diseases leading to heart failure, such as congenital heart disease, cardiomyopathy, or severe arrhythmias, and avoiding factors such as excessive physical exertion, serve as effective means to prevent the occurrence and progression of heart failure.