Complete transposition of the great arteries (TGA) is the most common cyanotic congenital heart disease in the neonatal period, constituting 5%–7% of all congenital heart defects. The male-to-female ratio for the condition is between 4:1 and 2:1.
Pathological Anatomy
In normal anatomy, the conus beneath the pulmonary valve is well-developed, and the pulmonary artery is positioned anterosuperiorly to the left, connecting to the right ventricle. The conus beneath the aortic valve regresses, and the aorta is positioned posteroinferiorly to the right, connecting to the left ventricle.
In transposition of the great arteries, the conus beneath the aortic valve is fully developed and persists, leading to the aorta being positioned anterosuperiorly to the right, connecting to the right ventricle. In contrast, the conus beneath the pulmonary valve regresses, and the pulmonary artery is positioned posteroinferiorly to the left, connecting to the left ventricle. Beneath the aortic valve, a muscular connection exists between the conus and the tricuspid valve, while beneath the pulmonary valve, there is no conus structure, resulting in a fibrous connection to the mitral valve.
Common associated anomalies include atrial septal defects or patent foramen ovale, ventricular septal defects, patent ductus arteriosus, pulmonary artery stenosis, and coronary artery abnormalities.
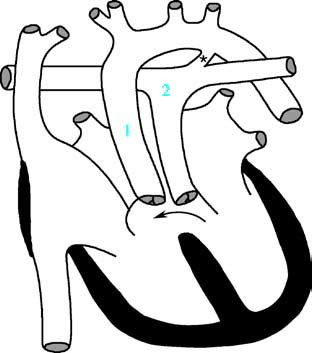
Figure 1 Schematic diagram of complete transposition of the great arteries
1, Aorta; 2, Pulmonary artery; the arrow indicates the ventricular septal defect; * marks the patent ductus arteriosus.
Pathophysiology
In complete transposition of the great arteries without additional defects, two parallel circulations are formed. Venous blood from the superior and inferior vena cava flows through the right heart and is ejected into the transposed aorta, supplying systemic circulation. Oxygenated pulmonary venous blood flows through the left heart and is ejected into the transposed pulmonary artery, returning to the lungs.
Survival in this condition depends on intracardiac communications (e.g., patent foramen ovale, atrial septal defect, ventricular septal defect) or extracardiac communications (e.g., patent ductus arteriosus, collateral vessels) to allow sufficient mixing of blood. The hemodynamic changes depend on the presence and nature of associated anomalies and are typically divided into three scenarios:
Complete Transposition of the Great Arteries with an Intact Ventricular Septum
The right ventricle is subjected to increased workload, leading to dilation and hypertrophy. As pulmonary vascular resistance naturally decreases after birth, the left ventricular pressure also decreases, and the ventricular septum often shifts toward the left ventricle. Blood mixing occurs only through a patent foramen ovale and ductus arteriosus, resulting in severe cyanosis and hypoxia.
Complete Transposition of the Great Arteries with a Ventricular Septal Defect
The presence of a ventricular septal defect allows more significant mixing of blood between the left and right circulations, reducing cyanosis. However, increased pulmonary blood flow may lead to congestive heart failure.
Complete Transposition of the Great Arteries with a Ventricular Septal Defect and Pulmonary Stenosis
The hemodynamics in this scenario resemble those seen in tetralogy of Fallot.
Clinical Manifestations
Cyanosis
Cyanosis often manifests early, with approximately half of cases presenting at birth and the vast majority developing it within the first month of life. Cyanosis gradually worsens with age and increased activity levels. It is systemic, but differential cyanosis may occur in cases of concomitant patent ductus arteriosus, with the upper limbs exhibiting more pronounced cyanosis than the lower limbs. Severe hypoxia can result in metabolic acidosis, hypoxic brain injury, and even life-threatening complications.
Congestive Heart Failure
Symptoms of congestive heart failure typically appear when the infant is 3–4 weeks old, including feeding difficulties, excessive sweating, tachypnea, hepatomegaly, and fine rales in the lungs.
Physical Examination
Infants often exhibit poor growth and development. At birth, heart murmurs may be absent, but a single, loud second heart sound resulting from the aortic valve closure is commonly heard due to its proximity to the chest wall. The presence of additional anomalies, such as large ventricular septal defects, patent ductus arteriosus, or pulmonary stenosis, can give rise to characteristic murmurs:
- In cases of patent ductus arteriosus, a continuous murmur may be heard at the second intercostal space along the left sternal border.
- In cases of ventricular septal defects, a holosystolic murmur can be heard at the third or fourth intercostal spaces along the left sternal border.
- In cases of pulmonary stenosis, a systolic ejection murmur may be heard at the upper left sternal border. Loud murmurs are often accompanied by thrills.
Patients with large ventricular septal defects commonly present with early-onset heart failure and pulmonary hypertension. In cases with pulmonary stenosis, cyanosis is more pronounced, and heart failure is less likely to occur.
Auxiliary Examinations
X-ray Examination
Due to the anteroposterior arrangement of the aorta and pulmonary artery, an anteroposterior chest X-ray may show a narrow mediastinal silhouette of the great vessels, with slight concavity in the pulmonary artery area and a small cardiac base, resulting in an "egg-shaped" heart appearance.
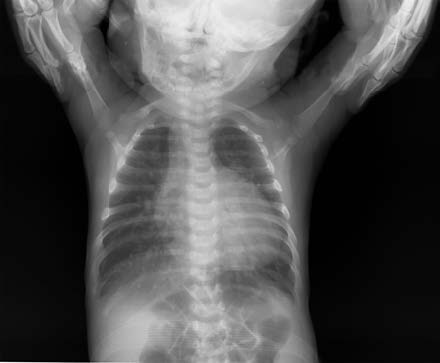
Figure 2 Chest X-ray of complete transposition of the great arteries (Frontal view)
The cardiac silhouette appears as an obliquely oriented "egg-shaped" heart with significant enlargement, predominantly involving the right and left ventricles. The pulmonary artery segment appears flattened, and there is pulmonary congestion in both lungs.
Progressive cardiac enlargement may be observed.
Increased pulmonary vascular markings are present in most patients. If pulmonary artery stenosis is also present, pulmonary vascular markings may appear reduced.
Electrocardiogram (ECG)
In the neonatal period, no specific abnormalities may be present. During infancy, findings may include right axis deviation, right ventricular hypertrophy, and occasionally, right atrial hypertrophy. In cases with significantly increased pulmonary blood flow, findings may also include normal or left axis deviation and hypertrophy of both the left and right ventricles.
Echocardiography
Two-dimensional echocardiography demonstrates normal atrioventricular connections and discordance between the ventricles and great arteries. The aorta and pulmonary artery are seen to be arranged in parallel, with the aorta typically positioned anterorightward, arising from the right ventricle, and the pulmonary artery positioned posteriorly to the left, arising from the left ventricle. Color and spectral Doppler echocardiography aids in determining the size and direction of intracardiac shunts and in detecting associated anomalies.
Cardiac Catheterization
In this condition, a catheter can be directly advanced from the right ventricle into the aorta. Right ventricular systolic pressure is equal to aortic pressure. Alternatively, the catheter may pass through the foramen ovale or an atrial septal defect into the left heart and subsequently into the pulmonary artery. Pulmonary artery oxygen saturation is higher than that of the aorta. During selective right ventricular angiography, the aorta can be visualized originating from the right ventricle. During left ventricular angiography, the pulmonary artery is observed originating from the left ventricle. Selective ascending aortography helps assess for the presence of coronary artery anomalies.
Treatment
Without intervention, approximately 90% of patients with complete transposition of the great arteries die within the first year of life. Once the diagnosis is confirmed, initial management focuses on correcting hypoxemia and metabolic acidosis. In cases where there is insufficient mixing due to a lack of adequate atrial septal or ventricular septal defects, prostaglandin E₂ is administered or a stent is placed to maintain ductal patency until surgical intervention.
Palliative Treatments
Balloon Atrial Septostomy (Rashkind Procedure)
For patients with severe hypoxia who are not candidates for immediate corrective surgery, balloon atrial septostomy or enlargement of an existing atrial septal defect is performed to allow significant mixing of blood at the atrial level. This increases arterial oxygen saturation and enables the patient to survive until definitive surgery can be achieved.
Pulmonary Artery Banding
In patients with large ventricular septal defects, pulmonary artery banding is performed within the first six months of life to prevent congestive heart failure and pulmonary vascular disease caused by pulmonary hypertension.
Definitive Surgical Treatments
Anatomic Correction (Jatene Procedure)
For patients with an intact ventricular septum, this procedure is performed within the first two weeks of life. It involves the arterial switch operation, during which the pulmonary artery and aorta are repositioned and the coronary arteries are reimplanted to achieve anatomic correction. Preoperative criteria include: left ventricular to right ventricular pressure ratio >0.85, left ventricular ejection fraction >45%, left ventricular end-diastolic volume >90% of normal, left ventricular posterior wall thickness >4 mm, and left ventricular wall stress <12 N/m. For those with ventricular septal defects, definitive surgery is performed within the first six months of life.
Physiological Correction (Senning or Mustard Procedure)
This surgery is typically performed between 1 and 12 months of age. It involves creating a baffle within the atria using pericardial tissue or atrial wall tissue. Systemic venous blood is redirected to the mitral valve and left ventricle, while pulmonary venous blood is redirected to the tricuspid valve and right ventricle. This creates atrioventricular and ventriculoarterial discordance, achieving a physiological correction.
Prognosis
Without treatment, approximately 90% of those with complete transposition of the great arteries die within the first year of life. In recent years, the widespread adoption of definitive surgical treatments has significantly reduced operative mortality rates. Patients who undergo successful surgery generally have favorable prognoses.