Pulmonary valve stenosis (PS) is a common form of congenital heart disease. Isolated pulmonary valve stenosis accounts for approximately 10% of congenital heart disease cases, while an additional 20% of congenital heart disease cases are associated with pulmonary valve stenosis. In a broader sense, pulmonary stenosis may involve the infundibular region, the valve itself, the main pulmonary artery, and the branches of the pulmonary artery.
Pathological Anatomy
Pulmonary valve stenosis is generally classified into two types:
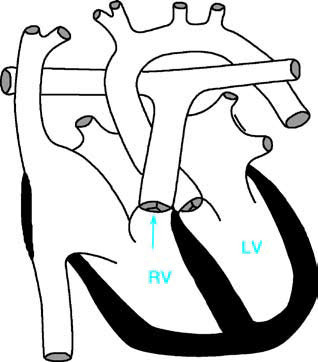
Figure 1 Schematic diagram of pulmonary valve stenosis
RV—Right Ventricle; LV—Left Ventricle; the arrow indicates the site of pulmonary valve stenosis.
Typical Pulmonary Valve Stenosis
Fusion of the cusps at the commissures of the three pulmonary valve leaflets restricts valve opening, resulting in a narrowed valve orifice. In bicuspid valve abnormalities, the commissures between the two fused leaflets limit valve function. In cases of unicuspid valve abnormality, the leaflets have no commissures and only a small central opening remains. The valve ring is usually normal, while post-stenotic dilation of the main pulmonary artery is evident, sometimes extending into the left pulmonary artery.
Dysplastic Pulmonary Valve Stenosis
This type of stenosis involves irregularly-shaped, thickened, or nodular valve leaflets. There is no fusion between the leaflets, but their opening and closing movements are restricted. Additionally, the valve ring is underdeveloped, and post-stenotic dilation of the pulmonary artery does not occur. Dysplastic pulmonary valve stenosis often has a familial component and frequently occurs in conjunction with Noonan syndrome.
Pathophysiology
The stenosis of the pulmonary valve impedes blood flow from the right ventricle to the pulmonary artery. In response to this obstruction, the right ventricle must generate higher systolic pressure to pump blood through the narrowed valve. The degree of increased systolic pressure correlates directly with the severity of the stenosis. Severe stenosis results in significant hypertrophy of the right ventricular wall, which may eventually cause reduced myocardial perfusion and lead to right-sided heart failure.
In fetal development, pulmonary valve stenosis causes hypertrophy of the right ventricular myocardium. Despite this, the fetal right ventricular output can often be maintained within normal limits, exerting minimal impact on fetal circulation. However, severe stenosis can lead to reduced right ventricular output, causing venous blood returning to the right atrium to flow predominantly through the foramen ovale or any atrial septal defect into the left atrium and ventricle. This flow pattern contributes to underdevelopment of the right ventricle.
Clinical Manifestations
Mild pulmonary valve stenosis is often asymptomatic. Moderate stenosis may remain asymptomatic until about two to three years of age, but older children may experience fatigue and dyspnea during physical exertion. Severe stenosis may lead to breathing difficulties and fatigue even with moderate physical activity, and in some cases, syncope or sudden death may occur. Occasionally, patients may experience chest pain or upper abdominal discomfort during activity, which may be caused by insufficient myocardial perfusion or arrhythmias due to limited cardiac output, indicating poor prognosis.
Growth and development are generally normal in affected individuals. Approximately half of the children affected may have rounded facial features. Cyanosis is typically absent, but the cheeks and fingertips may appear mildly dark red. Severe stenosis can sometimes cause cyanosis, mostly resulting from right-to-left shunting through the foramen ovale. In cases with large atrial septal defects, significant cyanosis, clubbing of fingers and toes, polycythemia, and red cell proliferation may be present. Squatting, which is commonly observed in conditions like tetralogy of Fallot, is rarely seen in pulmonary valve stenosis.
Prominent jugular venous pulsations may indicate severe obstruction, and the pre-systolic pulsation may also be palpable in the liver region.
The precordium may appear full, with diffuse pulsations. Right ventricular heave can often be palpated along the left parasternal region, indicating right ventricular hypertrophy. A grade 3 or louder systolic ejection murmur is frequently heard in the second or third left intercostal space near the sternum, with radiation to the left upper chest, precordium, neck, axilla, and back.
The first heart sound is typically normal. In mild to moderate stenosis, an early systolic click may be audible. Severe stenosis results in earlier clicks, sometimes coinciding with the first heart sound, causing the first heart sound to take on a metallic quality. The systolic click is caused by the sudden tightening of thickened, yet still elastic, valve leaflets during the onset of systole.
The second heart sound is split due to delayed closure of the pulmonary valve. The degree of splitting correlates proportionally with the severity of stenosis.
Auxiliary Examinations
X-ray Examination
In cases of mild to moderate pulmonary valve stenosis, the heart size appears normal. In severe cases, if cardiac function remains adequate, the heart may only exhibit mild enlargement. In cases of heart failure, significant cardiac enlargement is observed, primarily due to right ventricular and right atrial dilation. Post-stenotic dilation of the pulmonary artery is a characteristic feature of this condition, sometimes extending into the left pulmonary artery. In infants, such dilation is often less pronounced.
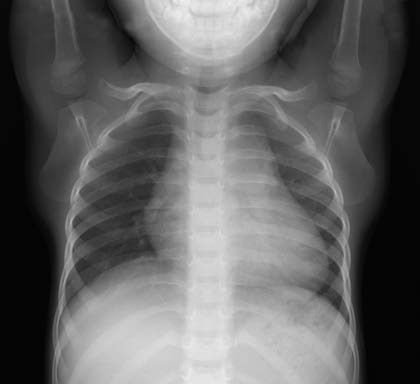
Figure 2 X-Ray of pulmonary valve stenosis (Frontal view)
Significant cardiac silhouette enlargement, primarily due to right ventricular enlargement. Prominent pulmonary artery segment and sparse vascular markings in both lungs.
Electrocardiography (ECG) Examination
Findings typically include right axis deviation, right atrial enlargement, elevated P waves, and right ventricular hypertrophy. Right precordial leads often reveal tall R waves. Severe stenosis may result in T-wave inversion and ST-segment depression.
Echocardiography
Two-dimensional echocardiography provides visualization of the pulmonary valve, including the number of leaflets, their thickness, mobility during systole, and post-stenotic dilation. Doppler echocardiography measures blood flow velocity at the pulmonary valve orifice as well as the pressure gradient between the right ventricle and the pulmonary artery, offering reliable assessment of the severity of the stenosis. Color Doppler imaging can also evaluate the presence of any shunts at the atrial level.
Cardiac Catheterization
Right ventricular pressure is significantly elevated, sometimes even exceeding systemic circulatory pressure in severe cases. Pulmonary artery pressure is notably reduced. Continuous pressure curves recorded as the catheter exits the pulmonary artery into the right ventricle show a distinct pressure gradient without a transition zone. Right ventricular angiography typically reveals the "jet phenomenon" at the pulmonary valve orifice, along with evidence of leaflet thickening and/or dysplasia and post-stenotic dilation of the main pulmonary artery trunk.
Treatment
When right ventricular systolic pressure exceeds 50 mmHg, myocardial damage is considered likely, indicating the need for intervention to relieve the obstruction. Balloon valvuloplasty performed via catheterization is the preferred treatment for most pediatric cases. For severe pulmonary valve stenosis, characterized by right ventricular systolic pressure exceeding systemic pressure, balloon valvuloplasty is also considered the first-line treatment. In cases where balloon valvuloplasty is not suitable, surgical valvulotomy is required.
Prognosis
The surgical outcomes for pulmonary valve stenosis are highly effective, with a high success rate. Severe pulmonary valve stenosis may be accompanied by infundibular stenosis, but in most cases, right ventricular infundibular hypertrophy resolves spontaneously after relief of the pulmonary valve stenosis. Postoperative follow-up is necessary to monitor valve function and to identify potential risks of pulmonary valve regurgitation or re-stenosis in a timely manner.