Ventricular septal defect (VSD) is the most common congenital heart disease, accounting for approximately 50% of all cases. About 40% of patients with VSD have associated cardiovascular anomalies.
Pathological Anatomy
Ventricular septal defects include several types. Based on the location of the defect within the ventricular septum and its relationship to the atrioventricular and semilunar valves, VSD can be classified into the following three types:
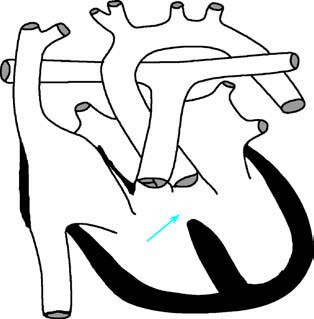
Figure 1 Schematic diagram of ventricular septal defect
The arrow indicates the location of the ventricular septal defect.
Perimembranous Type
This is the most common type, accounting for 60%–70% of cases. The defect is located in the membranous septum beneath the ventricular crest and can extend to the inlet, outlet, or trabecular regions.
Muscular Type
This type accounts for 10%–20% of cases. The defect is surrounded entirely by muscle tissues, with the membranous septum remaining intact. It may occur in the trabecular muscle region, inlet muscle region, or outlet muscle region.
Subarterial (Doubly Committed) Type
This type is less common and is more frequently observed in individuals of East Asian descent compared to Western populations. The defect is located in the right ventricular outflow tract, with its upper margin formed by the conjoined annuli of the aortic and pulmonary valves.
Pathophysiology
The physiological consequences of VSD depend on the size of the defect and pulmonary vascular resistance. After blood flows into the left ventricle from the left atrium, a portion enters the aorta and systemic circulation as effective circulation, while another portion is diverted through the ventricular septal defect into the right ventricle and pulmonary circulation as ineffective circulation. This results in an imbalance between systemic and pulmonary blood flow, with pulmonary circulation exceeding systemic circulation. VSD can present in three scenarios:
Small VSD (Roger's Disease)
The defect diameter is less than 5 mm, or the defect area is less than 0.5 cm²/m² of body surface area. The shunting volume is minimal, and hemodynamic changes are insignificant. Symptoms are typically absent.
Moderate VSD
The defect diameter ranges between 5 mm and 10 mm, or the defect area is 0.5–1.0 cm2/m2. The shunting volume is significant, and pulmonary blood flow may exceed systemic blood flow by a factor of 1.5 to 3. However, due to the substantial reserve capacity of the pulmonary vascular bed, pulmonary arterial pressure and pulmonary vascular resistance may remain normal over an extended period.
Large VSD
The defect diameter exceeds 10 mm, or the defect area is greater than 1.0 cm2/m2. A large shunting volume significantly increases pulmonary blood flow. When the capacity of the pulmonary vascular bed is exceeded, volume-overload pulmonary hypertension occurs. Persistent reactive vasoconstriction of small pulmonary arteries becomes evident, followed by progressive thickening of the media and intima, resulting in lumen narrowing and obstruction. With ongoing pulmonary vascular disease, irreversible obstructive pulmonary hypertension develops. When right ventricular systolic pressure surpasses that of the left ventricle, the left-to-right shunting reverses to bidirectional or right-to-left shunting, resulting in Eisenmenger syndrome.
Clinical Manifestations
Small defects are often asymptomatic, with normal activity tolerance and growth and development. On physical examination, a loud pansystolic murmur is typically heard at the third or fourth intercostal space along the left sternal border, often accompanied by a palpable thrill. The second heart sound over the pulmonary valve may be normal or slightly accentuated.
Moderate defects result in an increased left-to-right shunting volume. Affected children may exhibit growth retardation, failure to gain weight, a wasted appearance, feeding difficulties, fatigue, shortness of breath, and excessive sweating. Recurrent respiratory infections may occur, which can lead to congestive heart failure. In some cases, an enlarged pulmonary artery may compress the recurrent laryngeal nerve, causing hoarseness. Cardiac pulsations become more active, and physical examination may reveal a grade III or higher rough pansystolic murmur at the third or fourth intercostal space along the left sternal border, with widespread radiation. A palpable systolic thrill is often present. In cases with significant pulmonary blood flow, a soft mid-diastolic murmur may be heard at the apex due to relative mitral stenosis.
Large defects with significant pulmonary hypertension, often seen in children or adolescents, result in markedly elevated right ventricular pressure and reversal of shunting from right to left. Cyanosis gradually develops and becomes increasingly pronounced. At this stage, heart murmurs become softer, while the second heart sound over the pulmonary valve becomes significantly accentuated.
Auxiliary Examinations
X-ray Examination
For small defects, chest X-ray findings may show no significant changes, though there may be mild pulmonary artery elongation or prominence and slight pulmonary congestion. In moderate defects, the cardiac silhouette appears mildly to moderately enlarged, with enlargement of both the left and right ventricles, predominantly in the left ventricle. The aortic arch shadow appears smaller, while the pulmonary artery segment is dilated and the pulmonary field indicates congestion. In cases of large defects, the cardiac silhouette is moderately to severely enlarged, with notable right ventricular enlargement, accompanied by marked pulmonary artery prominence and significant pulmonary congestion. When pulmonary hypertension leads to Eisenmenger syndrome, the main pulmonary artery branches appear dilated, but peripheral pulmonary vascular markings are diminished, resembling withered branches. In such cases, the cardiac silhouette is often normal or slightly enlarged.
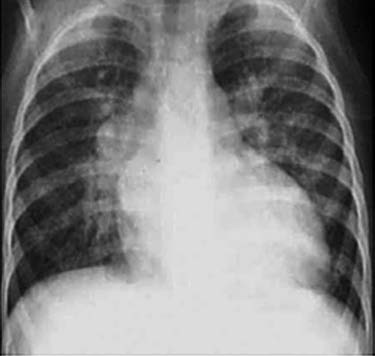
Figure 2 Chest X-ray of ventricular septal defect (Frontal view)
Electrocardiography (ECG) Examination
In small defects, ECG findings may be normal or show mild left ventricular hypertrophy. Moderate defects primarily demonstrate left ventricular diastolic overload, with elevated R waves and deep Q waves in leads V5 and V6, as well as upright, tall, sharp, and symmetrical T waves, indicating left ventricular hypertrophy. In large defects, there is evidence of biventricular hypertrophy or predominant right ventricular hypertrophy, which may be accompanied by myocardial strain patterns.
Echocardiography
Two-dimensional echocardiography can provide multi-plane views to identify the location, number, and size of the defects. Color Doppler imaging reveals the origin, location, number, size, and direction of the shunting jets. Spectral Doppler ultrasound allows the measurement of shunting velocities, determination of the trans-septal pressure gradient, and calculation of right ventricular systolic pressure and estimated pulmonary artery pressure. Pulmonary blood flow can also be calculated by measuring flow through the pulmonary valve and the mitral valve, while systemic blood flow can be estimated by measuring flow through the aortic valve and tricuspid valve. This information allows for the quantification of the left-to-right shunt volume.
Cardiac Catheterization
Cardiac catheterization and angiography are typically reserved for cases requiring additional information for comprehensive assessment. These techniques confirm the diagnosis, provide hemodynamic measurements, and enable accurate evaluation of pulmonary hypertension, calculation of pulmonary vascular resistance, and the measurement of shunt flow. Angiography illustrates the morphology and size of the cardiac chambers, as well as the ventricular-level shunt patterns. It also excludes the presence of other associated anomalies.
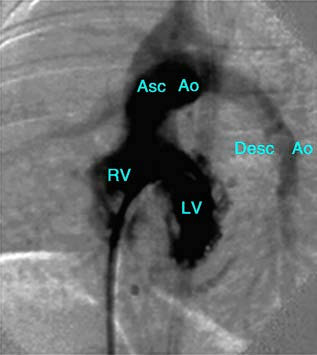
Figure 3 Left ventriculogram of ventricular septal defect
LV—Left Ventricle; RV—Right Ventricle; Asc Ao—Ascending Aorta; Desc Ao—Descending Aorta.
Treatment
Ventricular septal defect is prone to complications such as respiratory tract infections, congestive heart failure, and infective endocarditis, which require timely diagnosis and treatment. Subarterial and outlet muscular defects rarely close spontaneously and are associated with a risk of aortic valve prolapse and resulting aortic regurgitation, which necessitates early intervention. Surgical treatment is indicated under the following circumstances: moderate to large defects with a pulmonary-to-systemic blood flow ratio greater than 2:1, uncontrollable congestive heart failure, persistent pulmonary arterial pressure exceeding half of systemic pressure, and defects complicated by aortic valve prolapse or regurgitation. Open-heart surgical repair is the first-line approach for patients with associated anomalies or those unsuitable for interventional treatment. For selected cases, particularly membranous and muscular ventricular septal defects, transcatheter or transthoracic interventional closure can be an alternative, provided that the indications are strictly observed.
Prognosis
Surgical outcomes for ventricular septal defects are well-established, with extremely low operative mortality rates. Spontaneous closure occurs in approximately 20%–50% of membranous and muscular ventricular septal defects by the age of five, most commonly within the first year of life. Regular follow-up is recommended to assess the need for intervention.