Atrial septal defect (ASD) accounts for 5% to 10% of all congenital heart diseases and is one of the most common congenital heart conditions observed in adults. The male-to-female ratio is approximately 1:2.
Pathological Anatomy
Based on embryological development, atrial septal defects can be categorized into the following four types:
Primum Type
Also referred to as a type I atrial septal defect, this type accounts for approximately 15% of cases. The defect is located at the junction between the atrial septum and the endocardial cushion. It is often associated with clefts in the mitral or tricuspid valve, in which case it is referred to as a partial atrioventricular septal defect.
Secundum Type
This is the most common type, accounting for approximately 75% of cases. The defect is located in the central portion of the atrial septum at the site of the fossa ovalis and is also referred to as the central type.
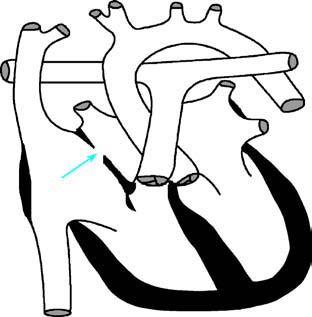
Figure 1 Schematic diagram of secundum-type atrial septal defect
The arrow indicates the atrial septal defect.
Sinus Venosus Type
This type accounts for about 5% of cases and can be further subdivided into the superior vena cava (SVC) type and the inferior vena cava (IVC) type. In the SVC subtype, the defect is located near the entrance of the superior vena cava, and anomalous drainage of the right upper pulmonary vein into the right atrium often occurs via this defect. In the IVC subtype, the defect is located near the entrance of the inferior vena cava and is typically associated with anomalous drainage of the right lower pulmonary vein into the right atrium. This subtype is often seen in scimitar syndrome.
Coronary Sinus Type
This type accounts for approximately 2% of cases. The defect is located between the superior portion of the coronary sinus and the left atrium, leading to a left-to-right shunt through the opening of the coronary sinus. This defect is often associated with persistent left superior vena cava, stenosis or atresia of the atrioventricular valves, complete atrioventricular septal defect, asplenia syndrome, or polysplenia syndrome.
Pathophysiology
Atrial septal defects result in a left-to-right shunt, with the shunting volume determined by the size of the defect, the pressure gradient between the atria, and particularly the compliance of the ventricles. During the early postnatal period, the wall thickness and compliance of the left and right ventricles are similar, leading to minimal shunting. As the individual ages, pulmonary vascular resistance and right ventricular pressure decrease, accompanied by thinning of the right ventricular wall. These changes reduce right ventricular filling resistance relative to the left ventricle, making it easier for blood to fill the right ventricle during diastole. Consequently, blood from the left atrium flows through the defect into the right atrium and increases the volume of blood entering the right heart. This results in right atrial and right ventricular enlargement due to the increased diastolic load. Increased pulmonary circulation leads to early dynamic pressure elevation and, in the long term, hypertrophy and intimal thickening of pulmonary arterioles, narrowing the vascular lumen. This can lead to obstructive pulmonary hypertension, which reduces or reverses the left-to-right shunt, potentially resulting in a right-to-left shunt.
Clinical Manifestations
The onset and severity of symptoms depend on the size of the defect. Small defects may remain asymptomatic and may only be discovered during physical examination, where a systolic murmur can be identified at the second to third intercostal space along the left sternal border. Larger defects result in a significant left-to-right shunt and pulmonary congestion, predisposing individuals to recurrent respiratory infections due to increased pulmonary circulation. In severe cases, early heart failure occurs. Moreover, systemic circulation may become insufficient, leading to features such as a lean body habitus, pallor, fatigue, excessive sweating, exertional dyspnea, and stunted growth and development.
Although most affected children show no evident signs during infancy, cardiac enlargement may become apparent over time, resulting in a prominent chest and active precordial pulsations. In cases with a significant shunt, a palpable thrill may also be present. Auscultation typically reveals the following four features:
- Accentuation of the first heart sound and intensification of the pulmonary component of the second heart sound.
- Fixed splitting of the second heart sound due to prolonged systolic ejection caused by increased right ventricular volume. Delayed closure of the pulmonary valve relative to the aortic valve occurs, persisting independent of respiratory phases.
- A soft grade II–III systolic ejection murmur is localized to the second intercostal space along the left sternal border. This murmur is caused by relative pulmonary valve stenosis due to increased right ventricular blood flow.
- In cases where the pulmonary circulation exceeds systemic circulation by a factor of two, a low-pitched, mid-diastolic murmur may be heard in the tricuspid valve area, caused by relative tricuspid stenosis.
As pulmonary hypertension progresses, left-to-right shunting gradually decreases. The second heart sound becomes accentuated, and its fixed splitting disappears. The systolic murmur shortens, and the diastolic murmur resolves. However, murmurs associated with pulmonary valve or tricuspid valve regurgitation may develop.
Auxiliary Examinations
X-ray Findings
For atrial septal defects with significant shunting, X-ray imaging can provide diagnostic value. The heart silhouette exhibits mild to moderate enlargement, primarily due to right atrial and right ventricular enlargement, with a cardiothoracic ratio greater than 0.5. The pulmonary artery segment is prominent, pulmonary vascular markings are increased, and the aortic shadow appears reduced. Fluoroscopy demonstrates a "hilar dance sign," characterized by pulsating light and dark changes in the main pulmonary artery and its branches in sync with cardiac motion. The cardiac silhouette may take on a pear shape. In cases of primum-type atrial septal defect accompanied by mitral valve cleft, enlargement of the left atrium and left ventricle may also be present.
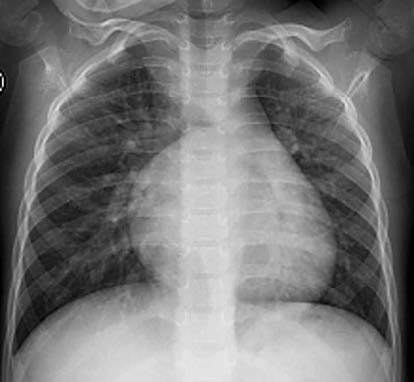
Figure 2 Chest X-ray of atrial septal defect (Frontal view)
Electrocardiography
Electrocardiography generally shows a sinus rhythm; in older patients, junctional rhythm or supraventricular arrhythmias may be observed. Most cases demonstrate right ventricular enlargement with an incomplete right bundle branch block pattern. There is right axis deviation, along with evidence of right atrial and right ventricular hypertrophy. PR interval prolongation may be noted, and QRS complexes in leads V1 and V3R often exhibit rSr' or rsR' patterns. In cases with significant left-to-right shunting, an R wave notch may be present. Primum-type atrial septal defect is frequently associated with left axis deviation and left ventricular hypertrophy.
Echocardiography
M-mode echocardiography shows enlargement of the right atrium and right ventricle, along with paradoxical motion of the interventricular septum. Two-dimensional echocardiography provides visualization of the location and size of the atrial septal defect. The use of color Doppler imaging enhances diagnostic accuracy and permits determination of the shunting direction. Spectral Doppler techniques allow estimation of shunt volume, right ventricular systolic pressure, and pulmonary artery pressure. In obese or older patients where transthoracic echocardiography may have poor acoustic windows, transesophageal echocardiography may be performed for diagnosis. Real-time three-dimensional echocardiography enables direct visualization of the defect from either the left or right atrial side, assessing the defect's overall morphology, its spatial relationship with adjacent structures, and its dynamic changes during the cardiac cycle.
Cardiac Catheterization
Routine use of cardiac catheterization is generally unnecessary. It may be performed when pulmonary hypertension, pulmonary valve stenosis, or anomalous pulmonary venous drainage is suspected. In such cases, the catheter can easily pass through the defect from the right atrium to the left atrium. In the presence of an atrial septal defect, oxygen saturation in the right atrium is higher than in the vena cava. Right ventricular and pulmonary artery pressures are normal or mildly elevated. Pulmonary vascular resistance and shunt volume can be calculated based on the obtained measurements. In cases with associated anomalous pulmonary venous drainage, investigations should identify the anomalously draining pulmonary veins. If necessary, cardiovascular angiography can be performed. Injecting contrast medium into the right upper pulmonary vein can demonstrate the rapid passage of contrast through the atrial septal defect from the left atrium to the right atrium.
Treatment
Large atrial septal defects carry a potential risk of heart failure and pulmonary hypertension. Surgical repair during childhood is preferred. Although conventional open-heart surgery involves significant trauma and extended recovery time, it remains the preferred method in cases of primum-type, sinus venosus-type, and coronary sinus-type atrial septal defects or when other anomalies are present. Under strictly defined indications, most secundum-type atrial septal defects can be repaired via catheter-based or transthoracic interventional closure after the age of two.
Prognosis
Surgical treatment of atrial septal defects yields excellent outcomes, with very low mortality rates. Small secundum-type defects may close spontaneously, often before the age of four, especially within the first year of life. Ongoing follow-up can help determine whether intervention is necessary in these cases.