Embryonic Development of the Heart
At the end of the second week of embryonic development, the cardiac primordia form two longitudinal tubular structures located on either side of the pharyngeal region on the ventral surface of the embryo. By day 22 of embryonic development, these two endothelial tubes gradually move toward the midline and fuse to form the primitive heart tube. Between days 22 and 24, the primitive heart tube begins to develop into various structures from the head to the tail, including the truncus arteriosus, bulbus cordis, ventricle, atrium, and sinus venosus. During this process, the heart tube undergoes bending and rotation. As the ventricles expand and elongate rapidly, they protrude ventrally, with the truncus arteriosus following and occupying the anterior position of the heart. Conversely, the atria and sinus venosus shift to the dorsal area above the ventricles. Four annular valve structures are fused together to create a fibrous framework, aligning the inflow and outflow pathways of the heart on the same horizontal plane.
By approximately day 29 of embryonic development, the external shape of the heart is essentially formed, though it remains a single tubular structure at this stage. The earliest division of the atrium and ventricle begins when the endocardial cushions grow from both the dorsal and ventral sides of the atrioventricular junction. These endocardial cushions fuse to form a central partition that separates the atrium and ventricle.
The division of the left and right atria begins around the end of the third week. A crescent-shaped septum, known as the primary atrial septum (septum primum), grows from the roof of the atrial chamber and extends toward the endocardial cushions, leaving a temporary opening called the foramen primum. Before the closure of the foramen primum, a second opening, the foramen secundum, forms in the upper portion of the septum primum, maintaining communication between the left and right atria. By the fifth to sixth week, a secondary crescent-shaped septum, known as the secondary atrial septum (septum secundum), begins growing adjacent to the right of the primary atrial septum. During its growth, the free edge of the septum secundum leaves an opening called the foramen ovale. The foramen ovale is aligned with the foramen secundum of the primary septum, allowing blood flow from the right atrium to the left atrium while preventing backflow due to the curtain-like structure of the primary atrial septum.
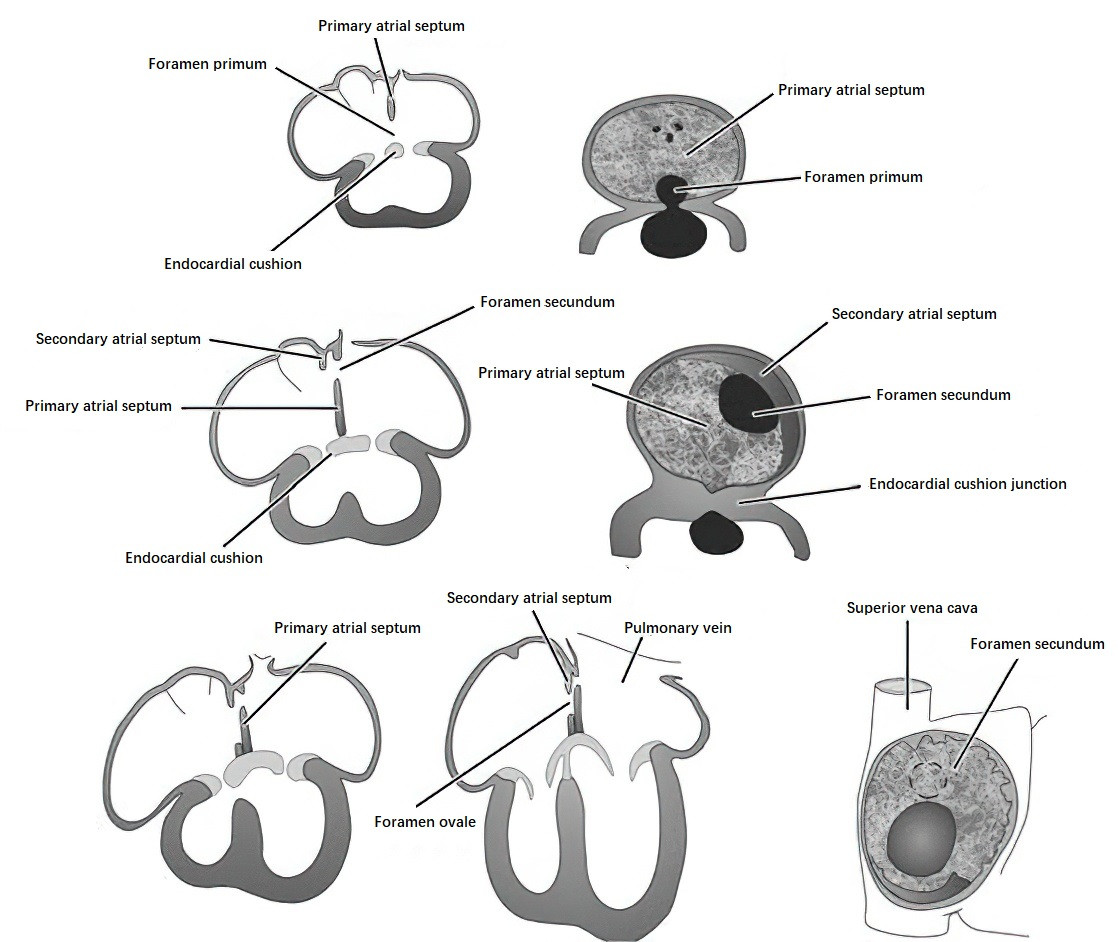
Figure 1 Schematic diagram of atrial septal development
Simultaneously, the partitioning of the ventricles occurs. A muscular septal primordium emerges from the base of the primitive ventricle and grows upward, dividing the ventricular chamber into left and right halves. By the seventh week, the membranous portion of the interventricular septum forms, completing the separation of the ventricles by closing the interventricular foramen. The formation of the interventricular septum involves three components:
- The muscular septum, which grows upward from the floor of the primitive ventricle to partially divide the left and right ventricles.
- The endocardial cushions, which grow downward and merge with the muscular septum to complete the septation.
- The contributions from the truncus arteriosus and bulbus cordis, which differentiate into the aorta and pulmonary artery, and their intermediate septum extends downward to contribute to the interventricular partitioning.
The mitral (bicuspid) and tricuspid valves are derived from the lateral and dorsal/ventral endocardial cushions at the atrioventricular junction.
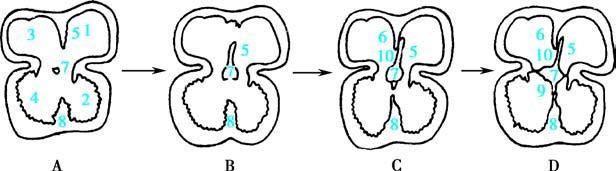
Figure 2 Schematic diagram of ventricular septal development
- Left atrium
- Left ventricle
- Right atrium
- Right ventricle
- Primary atrial septum
- Secondary atrial septum
- Endocardial cushion
- Muscular part of the ventricular septum
- Membranous part of the ventricular septum
- Foramen ovale
The primitive heart's outflow tract consists of the proximal conus region and the distal truncus arteriosus, collectively known as the conotruncal region, which is a frequent site of congenital cardiovascular anomalies. The interior of the bulbus cordis develops into two parts, forming the left and right ventricular outflow tracts, while the truncus arteriosus develops two opposing ridges that join along its central axis. This process divides the truncus arteriosus into the aorta and pulmonary artery. The spiral-shaped division of the truncal septum ensures that the pulmonary artery connects anteriorly and to the right with the right ventricle, while the aorta connects posteriorly and to the left with the left ventricle.
Starting around the fourth week, the primitive heart acquires circulatory function. By the eighth week, the partitioning of the heart is largely complete, resulting in a four-chambered structure. Most congenital cardiovascular malformations occur during this critical period of development.
Fetal-to-Neonatal Circulatory Transition
Normal Fetal Circulation
During fetal development, nutrient metabolism and gas exchange occur via diffusion across the placenta, facilitated by the connection between the umbilical vessels and the maternal circulation. Oxygenated blood from the placenta enters the fetus through the umbilical vein and reaches the liver. Approximately 50% of this blood flows into the liver to mix with portal venous blood, while the remainder bypasses the liver via the ductus venosus and enters the inferior vena cava, where it mixes with venous blood from the lower body. After entering the right atrium, the mixed blood from the inferior vena cava (predominantly oxygenated) is directed by the valve of the inferior vena cava, with about one-third passing through the foramen ovale into the left atrium. This flows into the left ventricle and then into the ascending aorta, primarily supplying the heart, brain, and upper extremities. The remaining blood enters the right ventricle.
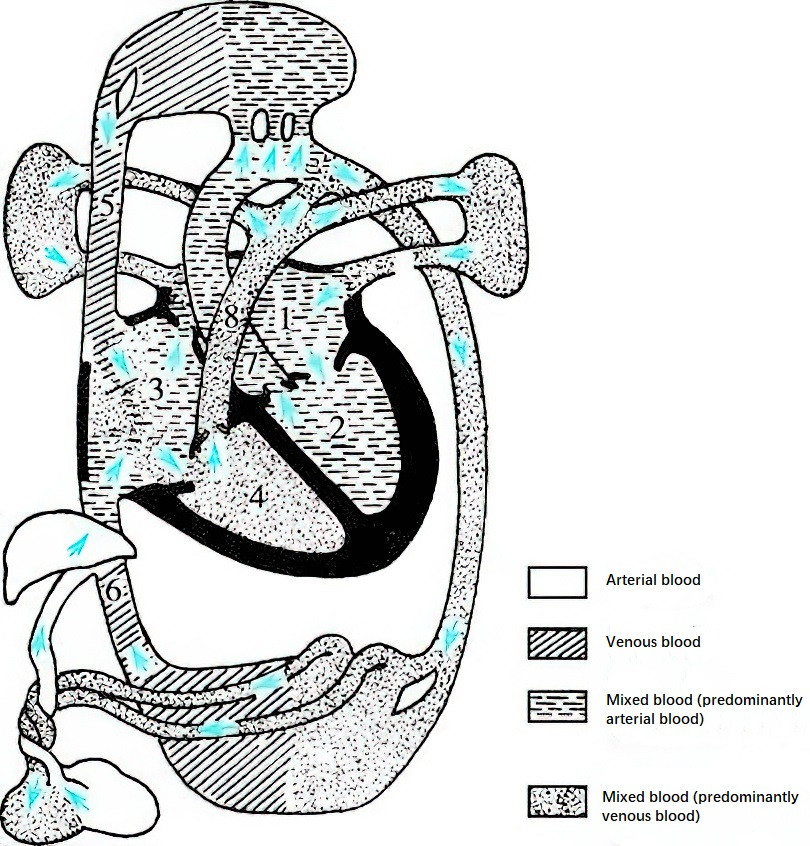
Figure 3 Characteristics of normal fetal circulation
- Left atrium
- Left ventricle
- Right atrium
- Right ventricle
- Superior vena cava
- Inferior vena cava
- Ascending aorta
- Main pulmonary artery
Deoxygenated blood returning from the upper body via the superior vena cava flows into the right atrium and predominantly passes into the right ventricle, mixing with blood from the inferior vena cava before entering the pulmonary artery. Since the fetal lungs are nonfunctional and compressed, only a small amount of blood flows into the lungs and returns via the pulmonary veins to the left atrium. Approximately 80% of the blood in the pulmonary artery bypasses the lungs through the ductus arteriosus, where it mixes with blood from the ascending aorta and enters the descending aorta (predominantly deoxygenated). This blood supplies the abdominal organs and lower limbs and circulates back to the placenta through the umbilical arteries for oxygen and nutrient exchange. Therefore, blood supplying the brain, heart, liver, and upper extremities during the fetal period is significantly more oxygen-rich than the blood supplying the lower body. During this time, the right ventricle not only overcomes systemic resistance but also handles a volume workload greater than the left ventricle.
Postnatal Changes in Circulation
After birth, the umbilical vessels are cut, and respiration is established as the alveoli expand. The muscular layer in the walls of the pulmonary arterioles gradually regresses, and the walls thin and dilate, leading to a decrease in pulmonary circulation pressure. Blood flow to the lungs from the right ventricle via the pulmonary artery increases, which also elevates pulmonary venous return to the left atrium. As a result, left atrial pressure rises. When left atrial pressure exceeds right atrial pressure, the foramen ovale functionally closes. By 5–7 months postnatally, the foramen ovale anatomically closes in most cases.
Increased oxygen levels due to independent respiration stimulate the contraction of the smooth muscle in the ductus arteriosus. Simultaneously, the low-resistance placental circulation ends following umbilical cord ligation, causing a rise in systemic vascular resistance. The flow through the ductus arteriosus reverses to left-to-right shunting due to higher arterial oxygen pressure and decreased postnatal prostaglandin levels. This leads to the gradual closure of the ductus arteriosus, eventually resulting in obliteration and its transformation into the ligamentum arteriosum. In term neonates, approximately 80% exhibit functional closure of the ductus arteriosus within 10–15 hours of birth. Anatomical closure occurs in about 80% by three months after birth and in 95% by one year of age. The umbilical vessels also undergo complete obliteration, forming ligaments approximately 6–8 weeks after blood flow ceases.
Anatomical Characteristics
Neonatal myocardial fibers are thin and arranged loosely, with poorly developed interstitial connective tissue and elastic fibers. There are no fat cells, but a dense vascular network is present. As the child grows, myocardial fibers thicken and elongate, and elastic fibers and connective tissue gradually develop, reaching full maturity by adolescence. Similarly, the cardiac conduction system is not fully mature at birth.
The position of the heart in children changes with age. In neonates, the heart lies higher in the chest and tends to adopt a more horizontal orientation, with the apex beat located in the fourth intercostal space, lateral to the midclavicular line. After the age of two, the heart gradually shifts to a more oblique orientation, with the apex beat descending to the fifth intercostal space.
The relative size of the heart is larger in children than in adults. At birth, the heart weighs approximately 20–25 g, accounting for 0.8% of body weight. By 1–2 years of age, it reaches 60 g, accounting for 0.5% of body weight. At five years of age, the size is about four times that of a newborn heart, increasing to six times by nine years of age. By late adolescence, the heart reaches 12–14 times its neonatal size, which corresponds to adult levels. The growth rate of the heart varies during different stages of development, with the fastest growth occurring during the first year after birth and accelerating again between the ages of 7–9 and during puberty. Except during early adolescence, boys have heavier hearts than girls at all age stages.
At birth, the right and left ventricles are nearly equal in weight, with the right ventricular wall slightly thicker than the left. The right ventricle also forms part of the apex, and the thickness of both ventricular walls is approximately 0.5 cm. Postnatally, as pulmonary vascular resistance decreases and left ventricular workload increases, the left ventricle grows faster than the right in both weight and wall thickness, eventually forming the majority of the apex. By 5–6 years of age, the left ventricular wall is significantly thicker than the right. In older children, the left ventricular wall can grow to be twice as thick as the right, and the heart elongates more in the long axis than in the transverse axis, resulting in the heart transitioning from a spherical to an elliptical shape.
The elastic fibers in the great vessels of neonates are sparse, leading to reduced elasticity. As the vessels thicken and elastic fibers proliferate with age, the great vessels reach developmental maturity by approximately 12 years of age. In childhood, the coronary arteries and capillaries have relatively wider lumens than in adults, ensuring adequate blood supply to the myocardium and major organs such as the lungs, kidneys, intestines, and skin. Additionally, in children, the diameter of veins is nearly equal to that of arteries, whereas in adults, venous diameter can be up to twice that of arteries.
Physiological Characteristics
At birth, the vagus nerve in the heart is underdeveloped, while sympathetic innervation dominates. This results in a stronger influence of the sympathetic nervous system on cardiac function. By five years of age, autonomic regulation of the heart begins to resemble that of adults, reaching full maturity by the age of 10. Younger children, therefore, exhibit faster heart rates and blood flow velocities.
The blood circulation time is approximately 12 seconds in infants, 15 seconds in school-aged children, and 18–20 seconds in older children. Cardiac output per body weight or surface area is higher in children compared to adults. In 34-week fetuses, the output of the left and right ventricles is 284.71 ml/(kg·min) and 365.99 ml/(kg·min), respectively, with the right ventricle being dominant during the fetal period. Within a few days after birth, ventricular output rises to approximately 400 ml/(kg·min), with a noticeable increase in left ventricular output. This increase in cardiac output is primarily due to the higher oxygen consumption per kilogram of body weight. During the first three months after birth, cardiac output gradually approaches adult levels, averaging around 100 ml/(kg·min).