Bronchial asthma, commonly referred to as asthma, is a heterogeneous disease characterized by chronic airway inflammation and airway hyperresponsiveness. It is the most common chronic respiratory disease in childhood. Its primary features include chronic inflammation of the airways involving multiple inflammatory cells and mediators, which leads to heightened airway sensitivity to various stimuli. This results in widespread, variable, and reversible airflow limitation, and, over time, airway remodeling. Clinically, asthma presents with recurrent symptoms such as wheezing, shortness of breath, chest tightness, or coughing, often occurring or worsening at night or early in the morning. Most children experience symptom relief either spontaneously or with treatment.
Etiology
The onset of asthma results from the combined effects of genetic and environmental factors.
Genetic Factors
Asthma shows a significant genetic predisposition, with approximately 20% of patients having a family history of the condition. It is a complex, polygenic inherited disorder, with multiple susceptibility genes identified, including IL-4R, IL-13, IL6R, YLK40, PDE4D, and IL-33.
Environmental Factors
These include:
- Allergenic factors, such as indoor allergens (dust mites, animal dander, cockroach excrement, fungi) and outdoor allergens (pollen, grass particles).
- Food allergens, including milk, eggs, fish, shrimp, crab, and peanuts.
- Drug allergens, such as aspirin and antibiotics.
- Occupational allergens, including dust and organic volatile gases.
- Pathogens, particularly respiratory infections caused by viruses and Mycoplasma.
- Non-allergenic factors, including emotional changes, exercise, cold air, and obesity.
Pathogenesis
The pathogenesis of asthma involves immune-inflammatory mechanisms, neural regulation, and their interactions, although it is not yet fully understood.
Airway Immune-Inflammatory Mechanisms
Chronic Airway Inflammation
This involves the participation and interaction of multiple cells (eosinophils, mast cells, T lymphocytes, neutrophils, and airway epithelial cells, among others) and inflammatory mediators, cytokines, and chemokines. External allergens entering the body via inhalation, ingestion, or contact are endocytosed by antigen-presenting cells and activate T cells. Activated Th2 cells produce cytokines such as IL-4, IL-5, and IL-13, promoting B cells to generate large quantities of IgE (including antigen-specific IgE). IgE binds to the surface of mast cells or basophils, leading to sensitized cells.
Upon subsequent allergen exposure, two responses occur:
- Early-Phase Reaction: Within seconds, allergens cross-link IgE on the surface of sensitized mast cells or basophils, triggering the synthesis and release of histamine and prostaglandins. This process induces airway smooth muscle contraction, increased mucus secretion, and infiltration of inflammatory cells, resulting in asthma symptoms.
- Late-Phase Reaction: Occurring 4–6 hours later, this involves infiltration and aggregation of activated eosinophils, Th2 cells, neutrophils, macrophages, and basophils in the airway, with the secretion of inflammatory mediators such as histamines, leukotrienes, prostaglandins, active neuropeptides, eosinophil chemotactic factors, and transforming growth factors (TGF). This forms a complex network of interactions that perpetuates chronic airway inflammation.
Additionally, recent studies highlight the role of Th17 cells (pro-inflammatory) and Treg cells (anti-inflammatory) — two newly identified CD4+ T cell subtypes — in asthma pathogenesis. Immune imbalance between Th17 and Treg plays a critical role, although the exact mechanisms remain unclear.
Airway Hyperresponsiveness (AHR)
AHR refers to the heightened sensitivity of the airways to various stimuli, leading to exaggerated or premature contraction responses upon exposure. AHR is closely associated with chronic airway inflammation, which damages airway epithelium and exposes subepithelial nerve endings, resulting in increased reactivity. AHR reflects inflammation severity to some extent and can be quantitatively assessed through bronchial provocation testing.
Neural Regulation of the Airways
The bronchi are innervated by a complex autonomic nervous system that includes adrenergic, cholinergic, and non-adrenergic non-cholinergic (NANC) systems. In asthma, beta-adrenergic receptor function may be impaired, and cholinergic nerve tone can be heightened. The NANC system is further divided into inhibitory (i-NANC) and excitatory (e-NANC) subtypes.
- The i-NANC system releases neurotransmitters such as vasoactive intestinal peptide (VIP) and nitric oxide (NO), which relax bronchial smooth muscle.
- The e-NANC system releases neurotransmitters such as substance P (SP), neurokinins (NK), and calcitonin gene-related peptide (CGRP), which induce bronchial smooth muscle contraction.
An imbalance between these systems leads to bronchial smooth muscle contraction and contributes to disease pathophysiology.
Pathology and Pathophysiology
Chronic airway inflammation represents the fundamental pathological feature of asthma. Autopsy studies in children who died from asthma reveal lung tissue changes, including emphysema as well as mucus plugs filling both large and small airways. These mucus plugs consist of mucus, serum proteins, inflammatory cells, and cellular debris. Microscopically, findings include bronchial and bronchiolar epithelial cell shedding, infiltration of eosinophils and mononuclear cells into the airway walls, vascular dilation with microvascular leakage, thickened basement membranes, smooth muscle hyperplasia and hypertrophy, and proliferation of goblet cells and submucosal glands. Airway obstruction constitutes the core pathophysiological change in asthma and is caused by bronchial spasm, inflammatory swelling of the airway wall, mucus plug formation, and airway remodeling.
Bronchial Spasm
Acute bronchial spasm is part of the early-phase reaction in asthma, which is mediated by IgE-dependent release of mediators (type I hypersensitivity). This involves the release of histamine, prostaglandins, and leukotrienes from mast cells.
Inflammatory Swelling of the Airway Wall
Following antigen exposure in the airways, reduction in airway diameter occurs within 6 to 24 hours. This is a result of increased microvascular permeability and exudative changes, which thicken and swell the airway mucosa. This phase may or may not be accompanied by smooth muscle contraction and represents the late-phase reaction in asthma.
Mucus Plug Formation
Increased mucus production, characteristic of the late-phase reaction, leads to the formation of mucus plugs. In severe cases, extensive mucus plugging may obstruct small airways, causing severe respiratory distress and potentially leading to respiratory failure.
Airway Remodeling
Chronic and recurrent inflammation can lead to airway remodeling, which represents structural changes in the airways. These include airway wall thickening, extracellular matrix deposition, collagen accumulation, subepithelial fibrosis, smooth muscle proliferation and hypertrophy, myofibroblast proliferation, goblet cell metaplasia and hyperplasia of mucus glands, and thickening of the subepithelial reticular layer. Neovascularization also occurs.
Clinical Manifestations
Asthma typically presents with recurrent episodes of wheezing, coughing, shortness of breath, and chest tightness, with symptoms often being more pronounced at night or in the early morning. Triggers include upper respiratory tract infections, allergen exposure, vigorous physical activity, laughter, crying, and weather changes. Episodes often occur or worsen during autumn, winter, or seasonal transitions. Severe episodes may involve significant respiratory distress, prolonged expiratory phases with audible wheezing, sitting upright to aid breathing, anxiety, profuse sweating, and cyanosis.
On physical examination, expiratory wheezing may be heard during auscultation of the chest. In severe cases with widespread airway obstruction, wheezing sounds may diminish or disappear entirely, a condition referred to as "silent chest," which is considered a life-threatening sign of asthma. During asymptomatic intervals, there may be no clinical signs or symptoms, although expiratory wheezes might be audible during forceful exhalation in some patients.
Auxiliary Examinations
Pulmonary Function Testing
Pulmonary function testing plays a vital role in diagnosing asthma and assessing its severity and control status. It is particularly useful for children aged 5 years and older. Asthmatic children typically exhibit reversible obstructive ventilatory dysfunction with varying degrees of impairment, especially during acute exacerbations. Key parameters such as forced expiratory volume in one second (FEV1) and the FEV1/forced vital capacity (FVC) ratio are often reduced (normal FEV1 ≥ 80% of predicted; normal FEV1/FVC ≥ 80%). FEV1 < 80% of predicted and FEV1/FVC < 80% are considered critical indicators of airflow obstruction in pediatric asthma. When pulmonary function is impaired, bronchial dilation tests are used to assess the reversibility and severity of the obstruction. If pulmonary function tests are normal, bronchial provocation tests can help evaluate airway responsiveness. Peak expiratory flow (PEF) and its variability may also be monitored continuously for two weeks to aid diagnosis.
Allergen-Specific Testing
Skin prick tests using extracts of various inhalant or food allergens provide valuable diagnostic information for allergic diseases, indicating the individual’s sensitization to specific allergens. Allergen-specific IgE testing in serum can further confirm allergic status. Children suspected of asthma are typically evaluated for allergic sensitization using either skin prick testing or allergen-specific serum IgE measurements to identify relevant triggers.
Assessment of Airway Inflammation
Non-invasive methods such as sputum eosinophil count and fractional exhaled nitric oxide (FeNO) levels are used to evaluate eosinophilic airway inflammation.
Chest Imaging
Chest X-rays in asthmatic children are typically non-specific. For children with chronic asthma, signs of emphysema may appear. In cases involving diagnostic challenges, poor symptom control following treatment, or suspected comorbid respiratory diseases, imaging techniques such as chest X-rays or computed tomography (CT) are used to differentiate asthma from conditions such as pneumonia, tuberculosis, foreign body aspiration, or congenital respiratory abnormalities.
Bronchoscopy
Children with recurrent wheezing or coughing who fail to respond to standard asthma therapy, or those suspected of having comorbid conditions such as airway foreign bodies, endobronchial tuberculosis, or congenital respiratory abnormalities, may undergo bronchoscopy for further diagnostic clarification.
Diagnosis and Clinical Assessment
Diagnosis
The diagnosis of pediatric asthma primarily relies on clinical manifestations and evidence of reversible airflow obstruction, while excluding other diseases that could cause similar symptoms.
Diagnostic Criteria for Pediatric Asthma
These include:
- Recurrent episodes of wheezing, coughing, shortness of breath, and chest tightness, which are often associated with exposure to allergens, cold air, physical or chemical irritants, respiratory infections, exercise, or hyperventilation (e.g., laughing or crying). Symptoms typically occur or worsen at night or in the early morning.
- Scattered or diffuse wheezing sounds, predominantly during expiration, and prolonged expiratory phases can be heard in both lungs during episodes.
- Symptoms and signs improve with anti-asthma treatment or resolve spontaneously.
- Other diseases causing wheezing, coughing, shortness of breath, and chest tightness are excluded.
- For cases with atypical clinical manifestations (e.g., no obvious wheezing or wheezing sounds), at least one of the following must be met:
- A positive bronchodilator test, indicated by an increase in forced expiratory volume in one second (FEV1) of ≥12% following inhalation of 200–400 μg of a short-acting β2-agonist (e.g., salbutamol) within 15 minutes, or improvement in lung function after anti-inflammatory therapy (e.g., inhaled corticosteroids or leukotriene receptor antagonists) over 4–8 weeks, with an FEV1 increase of ≥12%.
- A positive bronchial provocation test.
- A diurnal variability in peak expiratory flow (PEF) ≥13% over two weeks of continuous monitoring.
Asthma can be diagnosed when criteria 1 through 4 are met, or criteria 4 and 5 are fulfilled.
Diagnostic Criteria for Cough Variant Asthma (CVA)
These include:
- Persistent coughing lasting longer than 4 weeks, often triggered or exacerbated by exercise, nighttime, or early morning episodes, with a predominance of dry cough without wheezing.
- No clinical signs of infection or failure to respond to prolonged antibiotic therapy.
- Improvement with diagnostic therapeutic anti-asthma treatment.
- Exclusion of other causes of chronic cough.
- A positive bronchial provocation test and/or a diurnal PEF variability ≥13% over two weeks of continuous monitoring.
- A personal or family history of atopy (in first- or second-degree relatives) or a positive allergen test.
Conditions 1 through 4 constitute the basic diagnostic criteria.
The diagnosis of asthma in children under six years of age remains a challenging clinical issue. Risk prediction models for asthma are often used to estimate the likelihood of asthma in young children with wheezing. For instance, the Asthma Predictive Index applies to children under three years of age with ≥4 wheezing episodes in the past year. A positive index is indicated by the presence of one major risk factor or two minor risk factors. Major risk factors include:
- A parental history of asthma.
- Physician-diagnosed atopic dermatitis.
- Evidence of sensitization to inhaled allergens.
Minor risk factors include:
- Evidence of sensitization to food allergens.
- Peripheral blood eosinophil counts ≥4%.
- Wheezing unrelated to common colds.
Clinical Assessment
Based on clinical manifestations, asthma can be categorized into acute exacerbation, chronic persistent, and clinical remission phases.
Acute exacerbation refers to a sudden onset of wheezing, coughing, shortness of breath, and chest tightness, or a significant worsening of pre-existing symptoms.
Chronic persistent refers to the presence of symptoms (e.g., wheezing, coughing, and chest tightness) with varying frequency and/or severity over the past three months.
Clinical remission is characterized by the absence of symptoms or signs, with lung function (FEV1 or PEF) ≥80% of the predicted value for at least three months, either with or without treatment.
Asthma requires further grading assessments, including severity of disease, level of symptom control, and severity of acute exacerbations. The severity of asthma is generally classified based on the treatment step required to achieve control, and assessments are typically performed after at least three months of standardized controller therapy.
- Mild asthma includes cases that achieve good control with step 1 or step 2 treatment.
- Moderate asthma includes cases that achieve good control with step 3 treatment.
- Severe asthma includes cases that require step 4 or step 5 treatment to achieve full control or cases that remain uncontrolled despite step 4 or step 5 treatment.
The Childhood Asthma Control Test (C-ACT) is commonly recommended for evaluating symptom control in children with asthma.
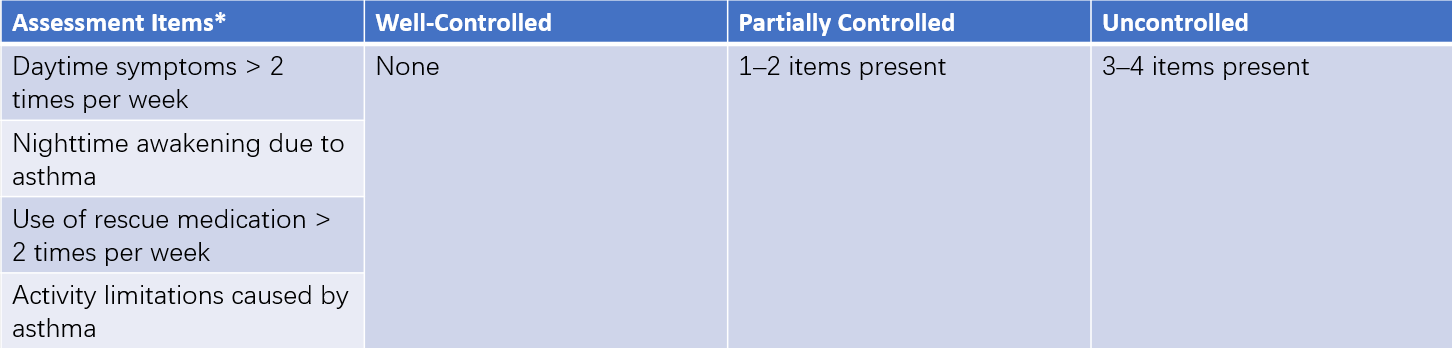
Table 1 Symptom control levels for children aged 6 years or older
Note: * Used to assess asthma symptoms over the past four weeks.
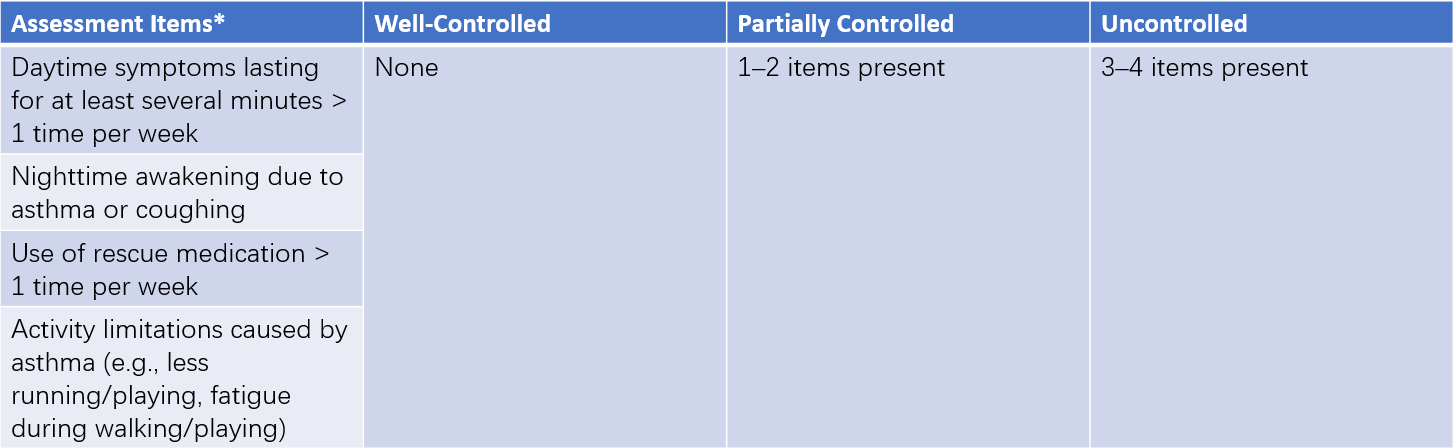
Table 2 Symptom control levels for children under 6 years
Note: * Used to assess asthma symptoms over the past four weeks.
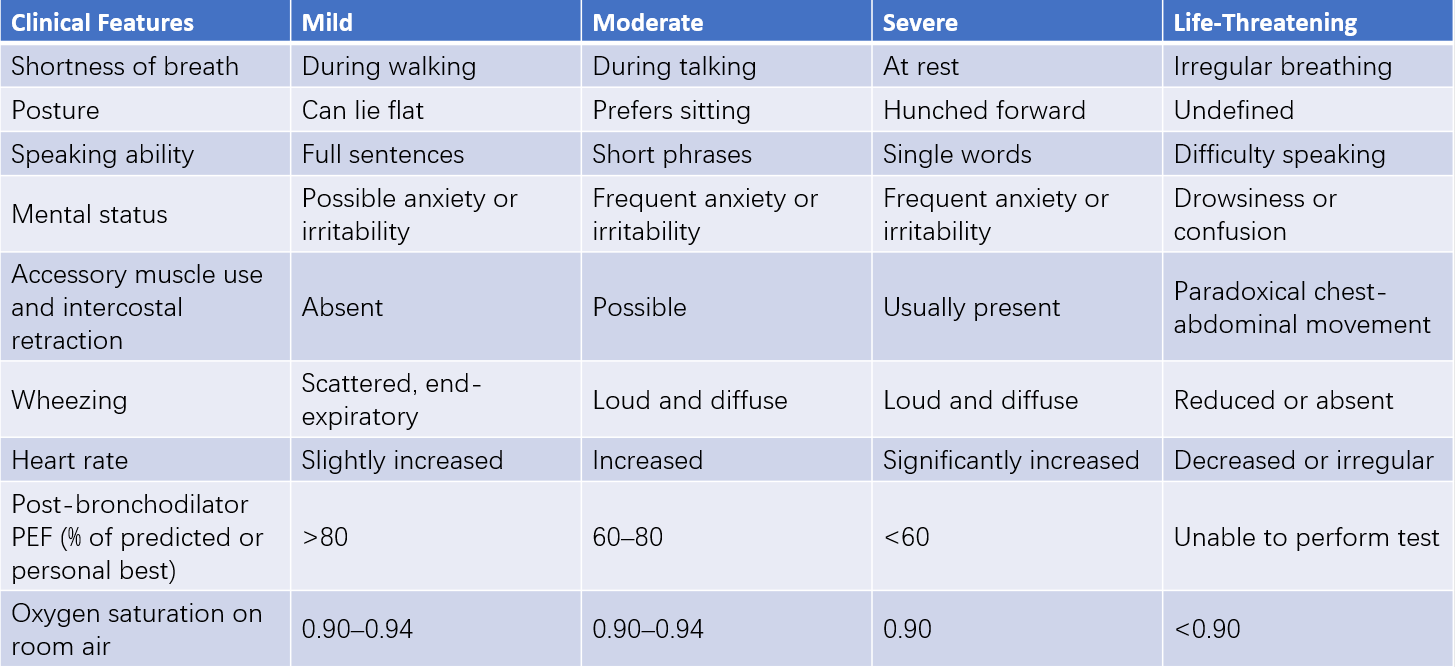
Table 3 Severity classification of acute asthma exacerbations in children aged 6 years or older
Notes:
Classification is based on the most severe characteristic observed.
Young children are more prone to hypercapnia (hypoventilation) compared to older children and adults.
PEF—Peak Expiratory Flow.
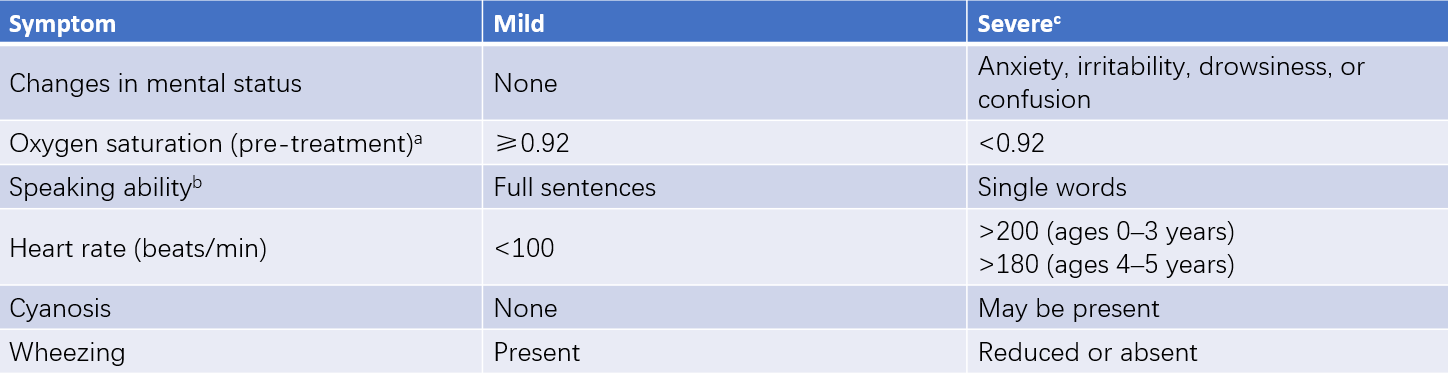
Table 4 Severity classification of acute asthma exacerbations in children under 6 years
Notes:
a, Oxygen saturation is measured before treatment with oxygen or bronchodilators.
b, Speaking ability needs to account for the normal language development process in children.
c, Severity classification is based on the presence of at least one severe indicator.
Differential Diagnosis
Before diagnosing pediatric asthma, other diseases causing recurrent cough and/or wheezing should be excluded. For children whose primary symptom is wheezing, it is important to differentiate asthma from conditions such as obliterative bronchiolitis, tuberculosis, airway foreign bodies, congenital abnormalities of the respiratory system, bronchopulmonary dysplasia, and congenital heart disease. For cough variant asthma (CVA), differentiation from diseases such as protracted bacterial bronchitis, recurrent viral respiratory infections, and gastroesophageal reflux should be considered.
Treatment
Goals of Asthma Treatment
The goals of asthma treatment include:
- Effective control of acute symptoms, with minimal or no persistent symptoms;
- Prevention of symptom exacerbation or recurrence;
- Maintenance of pulmonary function at or near normal levels;
- Prevention of irreversible airflow obstruction;
- Preservation of normal activity levels, including physical exercise;
- Avoidance of adverse drug reactions;
- Prevention of asthma-related mortality.
Asthma management should begin early and adhere to principles of long-term, continuous, standardized, and individualized treatment. Treatment during acute exacerbation focuses on anti-inflammatory and bronchodilator therapies to rapidly alleviate symptoms. For chronic persistent asthma, long-term anti-inflammatory treatment is essential to reduce airway hyperresponsiveness, prevent airway remodeling, and minimize exposure to risk factors, combined with self-care.
Chronic Persistent Asthma Treatment
Asthma medications are categorized into three groups: relief medications, control medications, and additional medications for severe asthma.
- Relief Medications: Used as needed during symptomatic episodes to quickly relieve bronchospasm and alleviate symptoms. These include short-acting β2-agonists (SABA), both inhaled and oral (e.g., salbutamol, terbutaline), inhaled short-acting muscarinic antagonists (SAMA), short-acting theophylline, and systemic corticosteroids.
- Control Medications: These are used daily on a long-term basis to control asthma through anti-inflammatory effects. The primary control medications include inhaled corticosteroids (ICS), systemic corticosteroids, leukotriene receptor antagonists (LTRA), long-acting β2-agonists (LABA), long-acting muscarinic antagonists (LAMA), sustained-release theophylline, and chromones such as sodium cromoglycate.
- Additional Medications for Severe Asthma: These include biologic targeted therapies, such as anti-IgE monoclonal antibodies and anti-IL-5 monoclonal antibodies.
Corticosteroids
Corticosteroids are the most effective medications for controlling airway inflammation in asthma. In chronic persistent asthma, the preferred route is inhalation due to its potent local anti-inflammatory effects and fewer systemic side effects. Commonly used ICS include budesonide, fluticasone propionate, and beclomethasone dipropionate. For severe persistent asthma that remains uncontrolled with high-dose ICS combined with LABA, low-dose oral corticosteroids may be added for maintenance therapy.
β2-Agonists
Inhaled LABA (e.g., formoterol, salmeterol) and inhaled SABA (e.g., salbutamol, terbutaline) are commonly used. Oral β2-agonists include bambuterol and procaterol.
ICS + LABA Combinations
ICS and LABA combinations provide synergistic anti-inflammatory and bronchodilatory effects, reducing the adverse effects of high-dose ICS. Examples include fluticasone propionate-salmeterol dry powder inhalers, budesonide-formoterol dry powder inhalers, and beclomethasone-formoterol aerosols.
Leukotriene Modulators
These include leukotriene synthesis inhibitors and leukotriene receptor antagonists (e.g., montelukast and zafirlukast). They are among the long-term control medications that can be used as monotherapy for mild asthma or as add-on therapy for moderate to severe asthma.
Theophylline
Sustained-release theophylline may be added for patients whose asthma is not adequately controlled with ICS or ICS+LABA.
Muscarinic Antagonists
Muscarinic antagonists have some bronchodilatory effects, though they are weaker and slower compared to β2-agonists. Their combination with β2-agonists produces complementary effects.
Biologic Targeted Therapies
These include treatments like anti-IgE monoclonal antibodies and anti-IL-4 receptor monoclonal antibodies, typically used as add-on therapies for severe asthma rather than monotherapy.
Allergen-Specific Immunotherapy (AIT)
AIT is currently the only treatment that may alter the natural course of allergic diseases. It is appropriate for asthma patients with clear allergen triggers and poorly controlled symptoms despite strict environmental control and medication.
Step-Up and Step-Down Treatment in Long-Term Pediatric Asthma Management
Standardized treatment is emphasized in pediatric asthma. Long-term treatment in children aged six years and older is divided into five steps, while it is categorized into four steps for children younger than six years. For newly diagnosed or uncontrolled asthma patients, treatment selection should follow the asthma control level, starting at step 2, step 3, or step 4. Treatment regimens should be reviewed every 1–3 months, adjusting as needed based on the level of symptom control. When symptoms are well controlled and pulmonary function remains stable for over three months, a step-down treatment may be considered. Partial control may require stepping up or intensifying therapy until control is achieved.
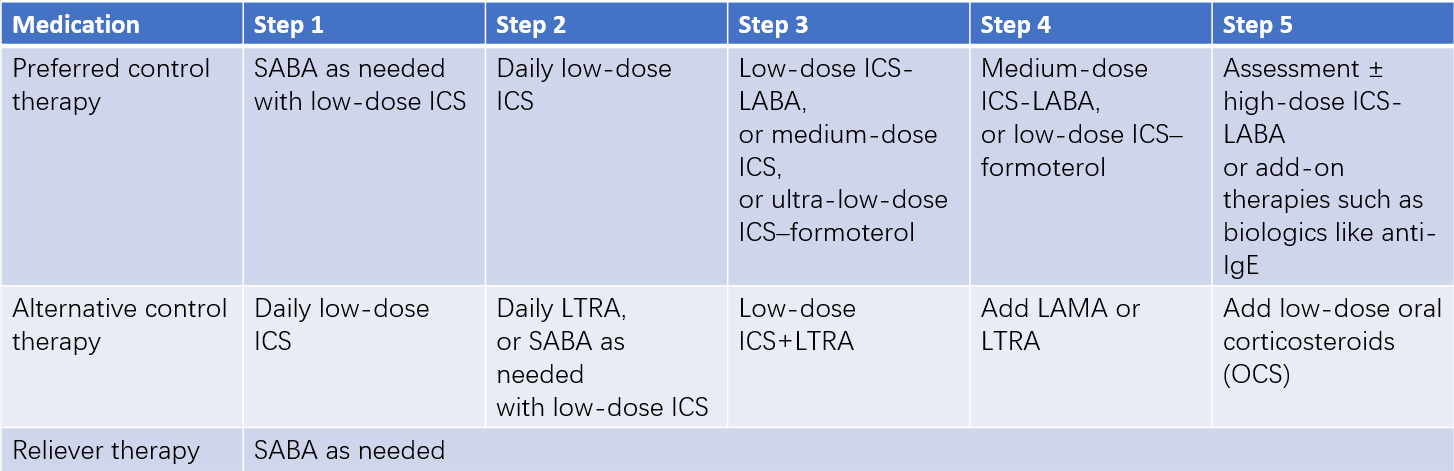
Table 5 Long-term asthma treatment regimens for children aged 6 years or older
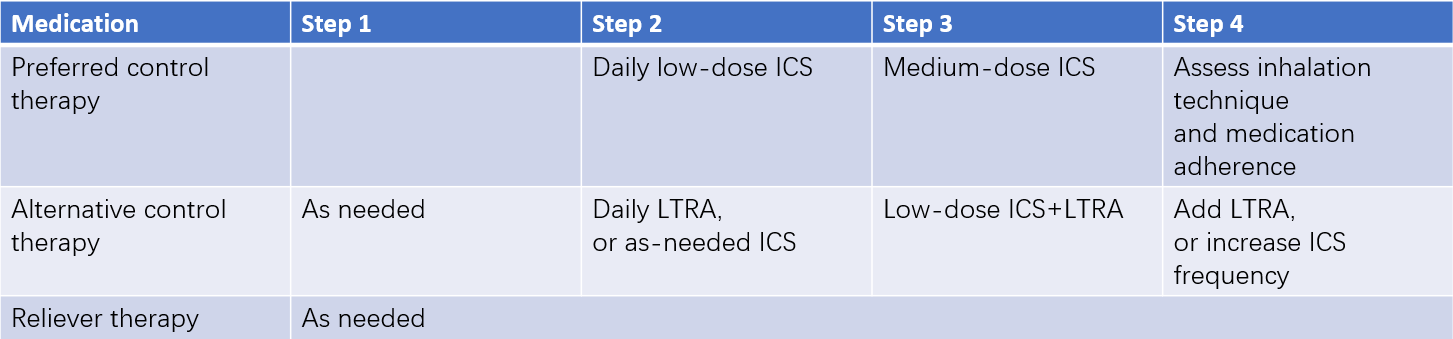
Table 6 Long-term asthma treatment regimens for children under 6 years
Treatment of Acute Asthma Exacerbations
Rapid and appropriate treatment is essential during acute exacerbations to promptly alleviate airway obstruction. Patients and/or parents should be guided to promptly administer inhaled SABA at the onset of exacerbation symptoms. If SABA fails to provide sufficient relief or if symptom improvement lasts less than four hours, hospital treatment is necessary. The main hospital treatments include:
Respiratory Support
Oxygen therapy should be provided for hypoxemia using nasal cannula or facemask oxygen delivery to maintain oxygen saturation above 0.94. For worsening symptoms, unresolved hypoxemia, or hypercapnia despite appropriate combination therapies, mechanical ventilation may be necessary.
β2-Agonists
Inhaled SABA remains the first-line treatment for acute exacerbations across all pediatric age groups, with effects lasting 4–6 hours. During severe exacerbations, inhalation may be repeated every 20 minutes during the first hour, followed by every 1–4 hours as needed.
Corticosteroids
Corticosteroids are the most effective option for controlling acute asthma exacerbations. Treatment routes depend on severity:
- Oral: Prednisone or prednisolone is administered at 1–2 mg/kg/day for 3–5 days. This route provides effective control with minimal side effects.
- Intravenous: Intravenous methylprednisolone (1–2 mg/kg/dose) or hydrocortisone sodium succinate (5–10 mg/kg/dose) can be given every 4–8 hours as needed. Steroid tapering is unnecessary for treatment durations under 10 days.
Muscarinic Antagonists
Inhaled SAMA may be added for moderate to severe exacerbations, particularly for patients with suboptimal responses to β2-agonists.
Magnesium Sulfate
Magnesium sulfate can help relieve severe exacerbation symptoms and is considered safe. Recommended dosage is 25–40 mg/kg/day (≤2 g/day), administered intravenously in 1–2 doses diluted in 20 ml of 10% glucose solution over 20 minutes or more, for 1–3 days as needed.
Short-Acting Theophylline
For patients whose exacerbations cannot be adequately controlled with the above therapies, aminophylline may be considered while closely monitoring for side effects, including ECG and serum drug levels. The recommended loading dose is 4–6 mg/kg (≤250 mg), administered intravenously over 20–30 minutes, followed by a maintenance infusion of 0.7–1 mg/kg/hour, adjusted based on age.
Management and Education
Avoidance of Risk Factors
Exposure to allergens should be minimized, with proactive treatment and elimination of infection sources. Triggering factors such as dust mites, respiratory infections, and pollen should also be addressed and managed.
Education and Management of Asthma
The education and management of children with asthma are essential for improving efficacy, reducing recurrence, and enhancing quality of life. Educating patients and their families on basic asthma prevention and treatment knowledge is critical in fostering active participation in asthma management, improving treatment adherence, avoiding various risk factors, consolidating treatment outcomes, and ultimately improving quality of life. It is also important for children and their families to learn how to use tools like the Childhood Asthma Control Test (C-ACT) and other asthma control questionnaires to assess asthma control levels accurately.
Diverse Educational Approaches
Educational initiatives for children with asthma and their families utilize various formats, including outpatient education, group activities (such as support groups or “asthma homes”), media campaigns (via radio, television, newspapers, popular science magazines, and books), and targeted education in collaboration with schools and community health organizations. These efforts aim to disseminate fundamental knowledge about asthma.
Prognosis
Although asthma cannot currently be cured, long-term standardized treatment allows most patients to achieve good or complete clinical control. In children, the prognosis is generally better than in adults, with a mortality rate of approximately 2–4 per 100,000 cases. Around 70%–80% of children experience symptom resolution as they grow older, though varying degrees of airway inflammation and hyperreactivity may persist. Approximately 30%–60% of children achieve complete control or spontaneous remission.