Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis. It can affect various organs in the body, but pulmonary tuberculosis is the most common form. Primary pulmonary tuberculosis is the most prevalent type of primary tuberculosis, resulting from the initial infection of the lungs by Mycobacterium tuberculosis. It is the primary form of lung tuberculosis in children. Tuberculous meningitis is the most severe manifestation of tuberculosis in children. In recent years, the incidence of tuberculosis has shown an increasing trend. The emergence of multidrug-resistant tuberculosis (MDR-TB) has become a serious challenge in the prevention and treatment of tuberculosis. Pediatric drug-resistant tuberculosis is often primarily resistant, acquired directly through transmission from patients with drug-resistant tuberculosis. This poses challenges with poor treatment outcomes and makes controlling the disease significantly more difficult.
This section provides a systematic overview of the different types of tuberculosis in children. Key diagnostic criteria and treatment principles for common forms of pediatric tuberculosis require particular attention.
Etiology
Mycobacterium tuberculosis belongs to the genus Mycobacterium. It is acid-fast, aerobic, gram-positive, and appears red by acid-fast staining. It reproduces slowly, taking 4–6 weeks to form colonies on solid culture media. Mycobacterium tuberculosis can be classified into four types: human, bovine, avian, and murine. The human and bovine types are primarily pathogenic to humans, with the human type being the main causative agent of human tuberculosis.
Epidemiology
Source of Infection
Patients with open pulmonary tuberculosis are the primary source of infection. Following 2–4 weeks of standardized chemotherapy, infectivity decreases as the bacterial load in sputum declines.
Routes of Transmission
The primary mode of transmission is through the respiratory tract. Inhalation of droplet nuclei or dust containing Mycobacterium tuberculosis can establish infection, leading to the formation of primary lesions in the lungs. Occasionally, the disease may spread via the digestive tract, resulting in primary lesions in the pharynx or intestines. Transmission via the skin or placenta is rare.
Susceptible Populations
All populations are generally susceptible to tuberculosis. Factors such as poverty, overcrowded living conditions, malnutrition, socioeconomic deprivation, and HIV infection contribute to the high prevalence of tuberculosis. The likelihood of disease development in children depends primarily on the following factors:
- Virulence and quantity of Mycobacterium tuberculosis.
- Immunological resistance of the host: Children with weakened immune systems due to conditions such as measles, pertussis, leukemia, lymphoma, or AIDS, or those receiving immunosuppressive therapies, are particularly susceptible to tuberculosis.
- Genetic predisposition: Genetic factors play a role in the susceptibility to tuberculosis. Concordance rates for tuberculosis are markedly higher in monozygotic twins than in dizygotic twins. Additionally, individuals of Asian descent (particularly Filipinos) have the highest incidence rates, whereas Caucasians have the lowest. Tall and thin individuals appear more susceptible compared to those who are shorter and broader. Research has also linked certain human leukocyte antigens (HLA) to tuberculosis risk, with individuals carrying HLA-BW35 being seven times more likely to develop the disease than the general pediatric population.
Pathogenesis
The development of tuberculosis in children following exposure to Mycobacterium tuberculosis is largely determined by host immunity, bacterial virulence, and bacterial load. Cellular immunity plays a particularly critical role. During Mycobacterium tuberculosis infection, the host develops both immunity and hypersensitivity responses. These manifestations are mediated by sensitized T-cells and represent two distinct aspects of the same cellular immune process.
Cell-Mediated Immune Response
Macrophages phagocytize and digest Mycobacterium tuberculosis, presenting specific antigens to helper T-lymphocytes (CD4+ cells). Macrophages, particularly dendritic cells, secrete IL-12 to induce polarization of CD4+ cells into Th1 cells, which secrete and release interferon-gamma (IFN-γ). IFN-γ enhances the activity of cytotoxic T lymphocytes (CTLs, CD8+ cells) and natural killer (NK) cells. This cascade of immune responses can ultimately eliminate Mycobacterium tuberculosis, but it may also cause destruction of host cells and tissues. When the immune response is insufficient to eradicate the bacteria, Mycobacterium tuberculosis may spread to lymph nodes via macrophages and lymphatic vessels.
Delayed-Type Hypersensitivity
This is an exaggerated immune response of the host to Mycobacterium tuberculosis and its metabolic products, mediated by T-cells. Macrophages act as effector cells in this response. The direct and indirect effects of delayed hypersensitivity lead to necrosis and caseous changes in tissues, potentially resulting in cavitation.
Following infection with Mycobacterium tuberculosis, the host usually acquires immunity, with 90% of cases remaining asymptomatic for life. Approximately 5% of individuals with inadequate immunity become symptomatic immediately, resulting in primary pulmonary tuberculosis. Another 5% develop disease later in life when immunity is compromised, leading to secondary pulmonary tuberculosis, which is the predominant type in adults.
During primary infection, Mycobacterium tuberculosis not only remains latent in chest lymph nodes but may also disseminate to other organs during the initial bacteremia, ultimately establishing latent foci that can later cause extrapulmonary tuberculosis.
Diagnosis
Early diagnosis is essential, including identifying the lesion, determining its nature, scope, and bacterial shedding status, as well as assessing its activity. This information serves as the basis for prevention and treatment.
Medical History
Infectious Symptoms
These include the presence or absence of symptoms such as prolonged low-grade fever, mild cough, night sweats, fatigue, loss of appetite, and weight loss.
History of Tuberculosis Exposure
Close contact with individuals with active tuberculosis, particularly among household members or classmates, holds significant diagnostic importance. Special attention is required for family history, as children younger than 5 years old are at high risk of exposure to active household pulmonary tuberculosis; younger age correlates with higher significance.
Vaccination History
BCG vaccination is an effective method for preventing tuberculosis infection and enhancing resistance against the disease. Examination of the left upper arm for a BCG vaccination scar is necessary.
History of Acute Infectious Diseases
Diseases such as measles and pertussis can temporarily suppress immunity, potentially activating latent tuberculosis lesions, worsening existing tuberculosis, or serving as a trigger for new infections.
Presence of Tuberculosis Hypersensitivity Symptoms
Symptoms such as erythema nodosum or phlyctenular conjunctivitis may indicate hypersensitivity to tuberculosis.
Tuberculin Skin Testing
Test Overview
The tuberculin test typically shows positive results 4–8 weeks after children are infected with Mycobacterium tuberculosis. This test reflects a delayed-type hypersensitivity reaction. The interpretation of results is based on the diameter of induration:
- <5 mm: Negative.
- 5–9 mm: Positive (+).
- 10–19 mm: Moderately positive (++).
- ≥20 mm: Strongly positive (+++).
Strongly positive reactions (++++) may involve additional manifestations such as edema, ulceration, lymphangitis, or concentric circle (double-ring) reactions.
For children with pronounced tuberculin hypersensitivity, such as those with phlyctenular conjunctivitis, erythema nodosum, or transient multiple hypersensitivity arthritis, 1 tuberculin unit of PPD is recommended to prevent localized overreaction and potential lesion reaction.
Clinical Relevance
Positive reactions may occur under these conditions:
- Following BCG vaccination.
- In older children without significant clinical symptoms, a positive reaction generally indicates prior infection with Mycobacterium tuberculosis.
- In infants, particularly unvaccinated infants, a positive reaction often suggests the presence of new active tuberculosis lesions. The younger the child, the greater the likelihood of active tuberculosis.
- Strong positive reactions indicate active tuberculosis.
A change from a negative to a positive reaction, or an increase in induration size from <10 mm to >10 mm with an increment greater than 6 mm, may indicate recent infection. The primary distinctions between BCG vaccination and natural infection-induced positivity are detailed in Table 1. Non-tuberculous mycobacteria infections may also cause positive PPD results.
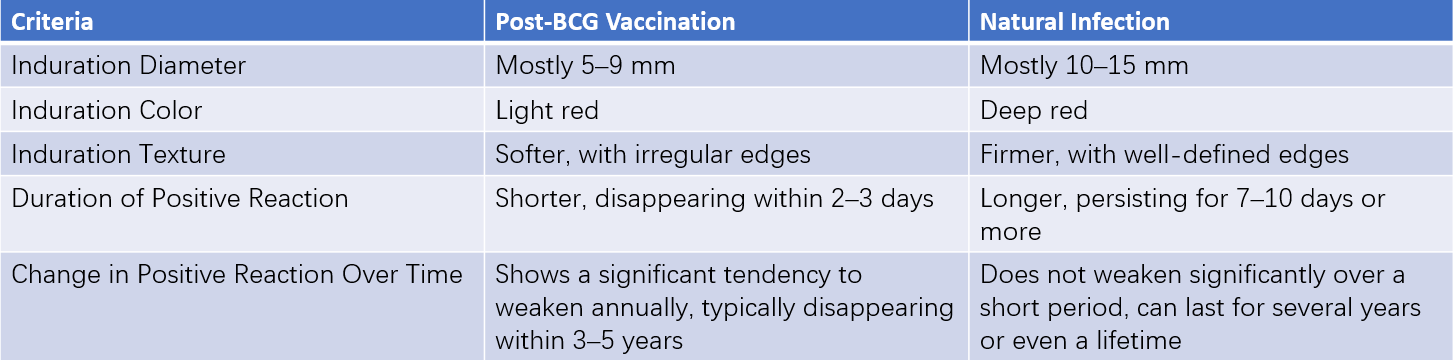
Table 1 Key differences between positive reactions from BCG vaccination and natural infection
Negative reactions may occur in these conditions:
- No prior infection with Mycobacterium tuberculosis.
- Early phase of delayed-type hypersensitivity (within 4–8 weeks after initial infection).
- False-negative reactions due to immunosuppression or compromised immune function, as seen in critically severe tuberculosis, acute infectious diseases (e.g., measles, varicella, rubella, pertussis), extreme physical debilitation (e.g., severe malnutrition, dehydration, or edema), immune suppressant administration (e.g., glucocorticoids), or primary/secondary immunodeficiencies.
- Technical errors or the use of expired tuberculin.
Laboratory Tests
Detection of Mycobacterium tuberculosis
The presence of Mycobacterium tuberculosis in sputum, gastric fluid (e.g., fasting gastric aspirates in infants), cerebrospinal fluid, serosal cavity fluid, or tissue samples serves as a definitive diagnostic tool.
Immunological and Molecular Diagnostic Techniques
Enzyme-Linked Immunosorbent Assay (ELISA)
This detects anti-Mycobacterium tuberculosis antibodies in serum, serosal cavity fluid, or cerebrospinal fluid.
Interferon-Gamma Release Assays (IGRAs)
Also known as T-Spot tests, IGRAs assess the immune response to specific Mycobacterium tuberculosis antigens by evaluating γ-interferon levels after exposure to these antigens. Alternatively, ELISPOT assays measure T-cells secreting γ-interferon. Positive IGRA results indicate tuberculosis infection but cannot distinguish between active tuberculosis and latent infection.
Molecular Biological Methods
Techniques such as nucleic acid hybridization and polymerase chain reaction (PCR) enable rapid detection of bacterial nucleic acids in clinical samples. Xpert MTB/RIF simultaneously detects Mycobacterium tuberculosis and rifampicin-resistance-associated genes (rpoB) with high sensitivity, especially in severely ill patients needing rapid diagnosis. This test is suitable for sputum, gastric aspirates, and cerebrospinal fluid. Next-generation sequencing (NGS) is also used for detecting Mycobacterium tuberculosis.
Erythrocyte Sedimentation Rate (ESR)
Accelerated ESR values may indicate tuberculosis activity.
Radiological Diagnosis
X-Ray Imaging
Chest radiography in both posteroanterior and lateral views provides information about the extent, nature, type, activity, and progression of tuberculosis lesions. Repeated imaging aids in distinguishing tuberculosis from other conditions and assessing treatment outcomes.
Computed Tomography (CT)
Chest CT is particularly valuable for diagnosing and differentiating pulmonary tuberculosis, especially in detecting hidden lesions. High-resolution thin-slice CT effectively demonstrates miliary tuberculosis within 2 weeks of onset and hilar or mediastinal lymph nodes ≥4 mm. CT provides greater sensitivity for identifying calcified lymph nodes compared to X-rays.
Other Auxiliary Examinations
Fiber Bronchoscopy
This facilitates the diagnosis of intrabronchial tuberculosis and bronchial lymph node tuberculosis.
Peripheral Lymph Node Aspiration and Smear Examination
Specific findings, such as tuberculous granulomas or caseous necrosis, support tuberculosis diagnosis and differential diagnosis.
Lung Biopsy via Percutaneous or Thoracoscopic Methods
This provides histopathological and etiological evidence, useful for diagnosing challenging or atypical cases.
Treatment
General Measures
Adequate nutrition with foods rich in protein and vitamins is important. Bed rest is recommended for individuals with significant tuberculosis toxicity symptoms or severe weakness. The living environment should have adequate sunlight and good ventilation. It is necessary to reduce the risk of concurrent infections with diseases such as measles or pertussis. Primary tuberculosis can usually be managed on an outpatient basis. Disease notification to health authorities is required, and follow-up visits for regular examinations during the treatment process are essential.
Antituberculosis Medications
The objectives of treatment include eradicating Mycobacterium tuberculosis in lesions and preventing hematogenous dissemination. The treatment principles involve early initiation of therapy, appropriate dosing, combination therapy, consistent administration, completion of the full treatment course, and phased treatment.
Categories of Antituberculosis Drugs
Bactericidal Agents:
- Fully bactericidal drugs, such as isoniazid (INH) and rifampin (RFP).
- Partially bactericidal drugs, such as streptomycin (SM) and pyrazinamide (PZA).
Bacteriostatic Agents: Common agents include ethambutol (EMB) and ethionamide (ETH).
Drugs for Drug-Resistant Strains of Mycobacterium tuberculosis
Additional agents are introduced based on first-line oral antituberculosis drugs:
- Group A: Fluoroquinolones such as moxifloxacin (Mfx), high-dose levofloxacin (Lfx), and gatifloxacin (GTFX).
- Group B: Injectable drugs, including kanamycin (Km), amikacin (Am), capreomycin (Cm), and streptomycin (S).
- Group C: Second-line oral bacteriostatic drugs such as ethionamide (Eto), prothionamide (Pto), and cycloserine (Cs). Linezolid and clofazimine are also included in this group.
- Group D: Optional drugs are further classified into:
- D1 (e.g., pyrazinamide, ethambutol, and high-dose isoniazid).
- D2 (e.g., newer drugs such as bedaquiline and delamanid, which are not recommended for pediatric use).
- D3 (e.g., para-aminosalicylic acid, imipenem-cilastatin, meropenem, amoxicillin-clavulanate, and thioacetazone).
Details on antituberculosis drug administration are presented in Table 2.
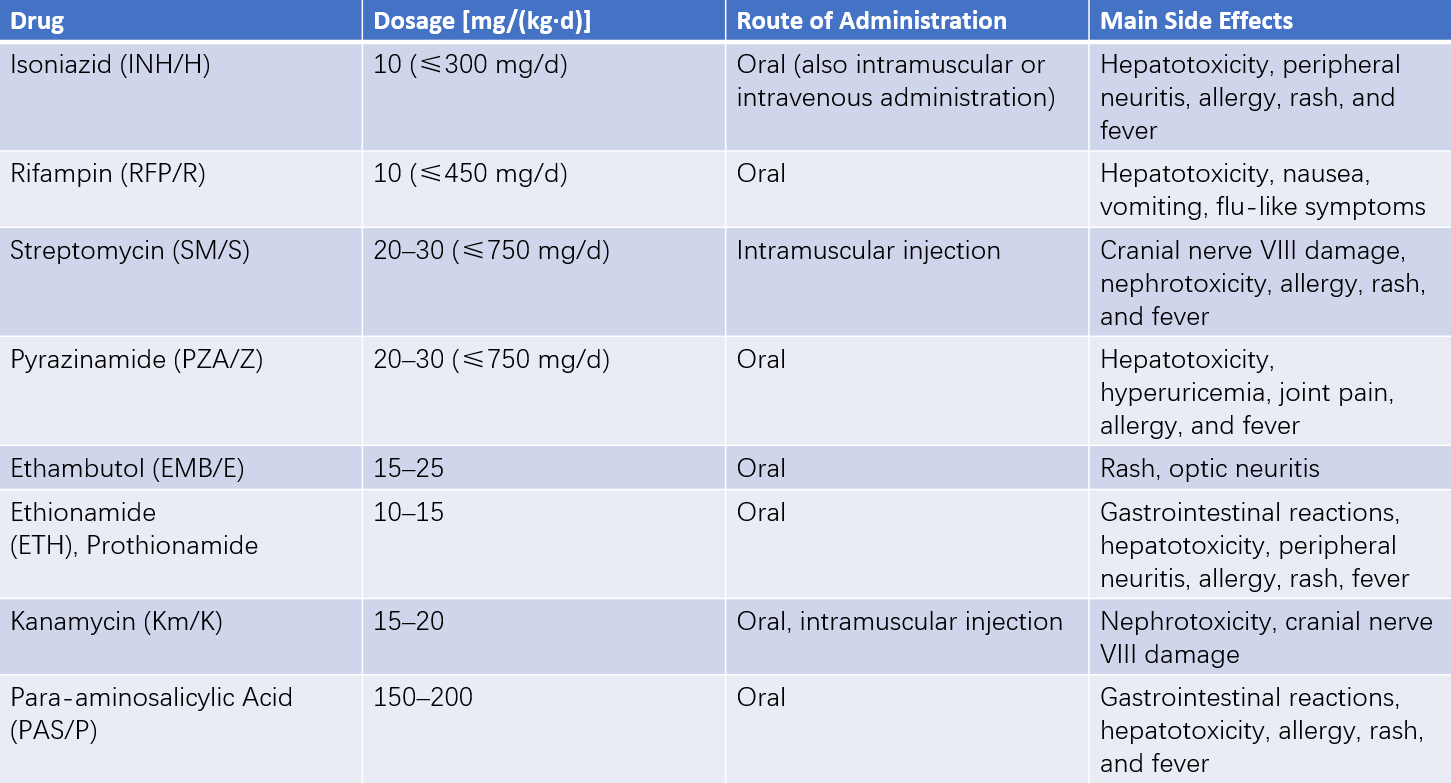
Table 2 Antituberculosis drugs for children
Antituberculosis Treatment Regimens
Standard Therapy
This is mainly used for primary pulmonary tuberculosis without significant clinical symptoms. INH, RFP, and/or EMB are administered daily for a duration of 9–12 months.
Two-Phase Therapy
This is employed for active primary pulmonary tuberculosis, acute miliary tuberculosis, and tuberculous meningitis.
In the intensive phase, 3–4 bactericidal drugs are combined to rapidly eliminate sensitive and metabolically active bacteria, as well as dormant bacteria, while preventing or reducing the emergence of drug-resistant strains. This phase typically lasts 3–4 months for long-term chemotherapy and 2 months for short-term chemotherapy.
In the continuation phase, 2 antituberculosis drugs are used to eradicate persistent bacteria, consolidate treatment effectiveness, and prevent relapse. This phase can last 12–18 months for long-term chemotherapy and 4 months for short-term chemotherapy.
Short-Course Therapy
A major advancement in modern tuberculosis treatment, directly observed treatment short-course (DOTS) is an important strategy recommended by the WHO to achieve cure. Short-course chemotherapy aims to rapidly eradicate Mycobacterium tuberculosis at various replication stages (both intracellular and extracellular), achieve early and lasting culture conversion, promote rapid lesion absorption, and minimize long-term relapse. Several options for short-course regimens lasting 6–9 months include:
- 2HRZ/4HR (numbers indicate months of treatment).
- 2SHRZ/4HR.
- 2EHRZ/4HR.
When PZA is excluded, the duration is extended to 9 months.
Treatment of Drug-Resistant Tuberculosis in Children
The principles of treatment are the same as for adults. Experience with long-term use of second-line antituberculosis drugs is limited in children, and thorough evaluation of drug risks and benefits is necessary before establishing treatment regimens.
Prevention
Control of Infection Sources
Patients with smear-positive Mycobacterium tuberculosis are the primary source of pediatric tuberculosis infections. Early detection and proper treatment of smear-positive patients are fundamental measures to prevent the spread of tuberculosis in children.
BCG Vaccination
BCG vaccination is an effective measure for preventing childhood tuberculosis. According to the national immunization plan, neonatal BCG vaccination is widely implemented across urban and rural areas. Vaccination is contraindicated under the following conditions:
- Congenital thymic aplasia or severe combined immunodeficiency, or individuals with HIV.
- Recovery phase of acute infectious diseases.
- Eczema or localized skin infections at the injection site, or systemic skin diseases.
- Positive tuberculin skin test.
Preventive Antituberculosis Treatment
Objectives:
- Prevention of active pulmonary tuberculosis in children.
- Prevention of extrapulmonary tuberculosis.
- Prevention of tuberculosis recurrence during adolescence.
Indications:
- Close household contact with individuals with open pulmonary tuberculosis.
- Tuberculin skin test positivity in unvaccinated infants and children under 3 years old.
- Recent conversion of tuberculin skin test from negative to positive.
- Positive tuberculin skin test with symptoms of tuberculosis.
- Positive tuberculin skin test in children who have recently developed measles or pertussis.
- Persistent positive tuberculin skin test in children requiring long-term glucocorticoid or other immunosuppressive therapy.
Administration:
- INH at 10 mg/kg daily (≤300 mg/day) for 6–9 months.
- Alternatively, INH at 10 mg/kg daily (≤300 mg/day) combined with RFP at 10 mg/kg daily (≤300 mg/day) for 3 months.