Neonatal sepsis refers to a systemic inflammatory response caused by the invasion, growth, reproduction, and toxin production of pathogens in a newborn's bloodstream. According to U.S. statistics, its incidence among live births ranges from 0.1% to 0.5%, with a mortality rate of 5%–10%. Lower gestational age and lower birth weight are associated with higher incidence and mortality rates. Common pathogens include bacteria, with occasional fungi or viruses. This section primarily addresses neonatal bacterial sepsis.
Etiology and Pathogenesis
Pathogenic Organisms
The pathogens vary depending on the region and time period. Staphylococci have long been the most common pathogens causing neonatal sepsis, followed by Gram-negative bacilli such as Escherichia coli. Advances in perinatal medicine, the establishment of Neonatal Intensive Care Units (NICUs), and the widespread survival of very low birth weight (VLBW) and extremely low birth weight (ELBW) infants have led to changes in the pathogen spectrum. Widespread hospital stays, use of intravenous catheters, endotracheal intubation, and broad-spectrum antibiotics have made coagulase-negative staphylococci (CNS) the leading pathogens identified in blood cultures. Escherichia coli remains significant, while Klebsiella species show an upward trend in developed cities, followed by Pseudomonas aeruginosa. Group B Streptococcus (GBS) and Listeria monocytogenes are common neonatal pathogens in developed countries like the U.S. and Europe.
Characteristics of the Neonatal Immune System
Non-Specific Immune Function
Barrier function is weak due to the thin and fragile stratum corneum of the skin and delicate, easily damaged mucosal linings. The umbilical stump remains incompletely closed, facilitating bacterial invasion into the bloodstream. Poor ciliary movement in the respiratory tract, high intestinal mucosal permeability, and the absence of secretory IgA (SIgA) increase susceptibility to respiratory and gastrointestinal infections. These factors also promote systemic infections and increase the risk of meningitis due to the underdeveloped blood-brain barrier.
Lymph nodes are underdeveloped, lacking sufficient capacity to filter and localize infections.
Complement components (e.g., C3, C5, and opsonins) are low in concentration, reducing the body's ability to opsonize certain bacterial antigens.
Neutrophil production and reserve are limited, with reduced chemotaxis, adhesion, and lysozyme content, resulting in deficient phagocytic and bactericidal functions, particularly in preterm infants.
Monocytes exhibit low production of cytokines such as granulocyte colony-stimulating factor (G-CSF) and interleukin-8 (IL-8).
Specific Immune Function
Neonatal IgG predominantly originates from maternal transfer via the placenta and correlates with gestational age. Premature infants, therefore, have lower IgG levels and are more prone to infections.
IgM and IgA, being large molecules, cannot cross the placenta and are present at very low levels in newborns, increasing susceptibility to Gram-negative bacterial infections.
T cells are largely naive, having had no prior exposure to specific antigens. Their cytokine production is inefficient, limiting their ability to effectively support B cells, macrophages, natural killer cells, and other immune cells in mounting a robust immune response.
Clinical Manifestations
Neonatal sepsis is classified into early-onset and late-onset types based on the timing of onset:
Early-Onset Sepsis (EOS)
Onset occurs within the first 3 days of life.
Infections are typically acquired before or during birth, with vertical transmission from the mother as the primary mode of acquisition. Gram-negative bacilli such as Escherichia coli predominate as causative pathogens.
Pneumonia frequently accompanies EOS, with rapid progression to multi-organ involvement and a case fatality rate as high as 5%–20%. It remains one of the leading causes of neonatal mortality. The prophylactic use of antibiotics during delivery in mothers at risk of infection has been shown to reduce neonatal mortality significantly.
Late-Onset Sepsis (LOS)
Onset occurs after the first 3 days of life.
Infections are typically acquired postnatally, through horizontal transmission involving environmental factors. Staphylococci and opportunistic pathogens are common culprits.
Focal infections, such as omphalitis or pneumonia, are often present. The mortality rate for LOS is generally lower than that for EOS.
Early symptoms and signs of neonatal sepsis are often atypical and non-specific, particularly in preterm infants. General signs include lethargy, poor feeding, reduced crying and movement, or even lack of feeding, crying, or movement altogether. Fever or hypothermia, failure to gain weight, or poor weight gain are also common. The following presentations strongly suggest sepsis:
- Jaundice: Occasionally the only clinical manifestation, with jaundice rapidly worsening, recurring after resolution, or, in severe cases, progressing to bilirubin encephalopathy.
- Hepatosplenomegaly: Often a late sign, usually presenting as mild-to-moderate enlargement.
- Bleeding Tendencies: Manifesting as petechiae, ecchymosis, gastrointestinal bleeding, or pulmonary hemorrhage.
- Shock: Featuring mottled skin, prolonged capillary refill time, hypotension, oliguria, or anuria.
- Other Symptoms: Vomiting, abdominal distension, toxic ileus, respiratory distress or apnea, and cyanosis.
- Possible Complications: The condition may be complicated by meningitis, pneumonia, necrotizing enterocolitis (NEC), septic arthritis, liver abscess, or osteomyelitis.
Auxiliary Examinations
Bacteriological Examinations
Blood Culture
Blood culture is conducted prior to the administration of antibiotics, with strict disinfection procedures during blood collection. Anaerobic bacterial culture is often conducted simultaneously in suspected cases of enterogenic infection to improve positive detection rates.
Cerebrospinal Fluid (CSF) and Urine Culture
Apart from culturing, CSF smears are examined for bacteria. Urine culture is best performed using suprapubic bladder aspiration to avoid contamination. A positive urine culture supports the diagnosis.
Others
Additional cultures, such as those from gastric aspirate and external ear secretions (within the first hour postpartum), throat swabs, skin swabs, umbilical stump secretions, or bronchoalveolar lavage fluid (for intubated infants), are useful. A positive result confirms bacterial colonization, though not definitive for a sepsis diagnosis.
Pathogen Antigen and DNA Detection
Techniques such as counter-immunoelectrophoresis and enzyme-linked immunosorbent assay (ELISA) are used to detect unknown pathogen antigens in blood, CSF, and urine using known antibodies. DNA probe-based molecular biology techniques may also assist in diagnosis.
Non-Specific Examinations
Peripheral Blood Profile
The total white blood cell (WBC) count may decrease (<5 × 109/L) or increase (≤3 days of age: WBC >30 × 109/L; >3 days of age: WBC >20 × 109/L). Due to significant fluctuations in the normal WBC range in neonates shortly after birth, interpretation should account for the infant’s postnatal age.
Cell Classification
The immature/total neutrophil (I/T) ratio is ≥0.16.
Platelet Count
Platelets <100 × 109/L.
C-Reactive Protein (CRP)
CRP is a sensitive acute-phase protein parameter that rises within 6–8 hours of acute infection, peaks at 8–60 hours, and rapidly decreases as the infection resolves. CRP ≥8 mg/L (based on peripheral blood methods) is considered abnormal.
Procalcitonin (PCT)
PCT rises earlier than CRP after bacterial infection and rapidly decreases with effective antibiotic treatment. It demonstrates high sensitivity and specificity, with PCT >2.0 µg/L generally indicating severe infection.
Interleukin-6 (IL-6)
IL-6 demonstrates 90% sensitivity and a negative predictive value >95%. It increases earlier than CRP following the onset of inflammation and returns to normal within 24 hours after resolution of inflammation.
Diagnosis
Confirmed Diagnosis of Sepsis
Clinical manifestations, along with any of the following, are required:
- Growth of pathogenic bacteria in blood cultures or sterile body cavity fluid cultures.
- For blood cultures yielding opportunistic pathogens, confirmation requires the same bacteria to be isolated from a second blood sample, a sterile body cavity, or the tip of a catheter.
Clinical Diagnosis of Sepsis
Clinical manifestations, along with any of the following, are required:
- Two or more abnormal findings on non-specific examinations.
- Positive detection of pathogen antigens or DNA in blood samples.
Treatment
Principles of Antibiotic Treatment
Early Administration
Antibiotics should be initiated as soon as neonatal sepsis is clinically suspected, without waiting for blood culture results.
Infection Source Clearance
Efforts should be made to eliminate the source of infection.
Intravenous and Combined Administration
Prior to pathogen identification, a combination of two antibiotics targeting Gram-positive and Gram-negative bacteria should be selected based on local epidemiology and patterns of antibiotic resistance. Once the pathogen is identified, antibiotics should be adjusted according to antimicrobial susceptibility testing. If clinical effectiveness is observed despite test results indicating resistance, substitutions are not necessary temporarily.
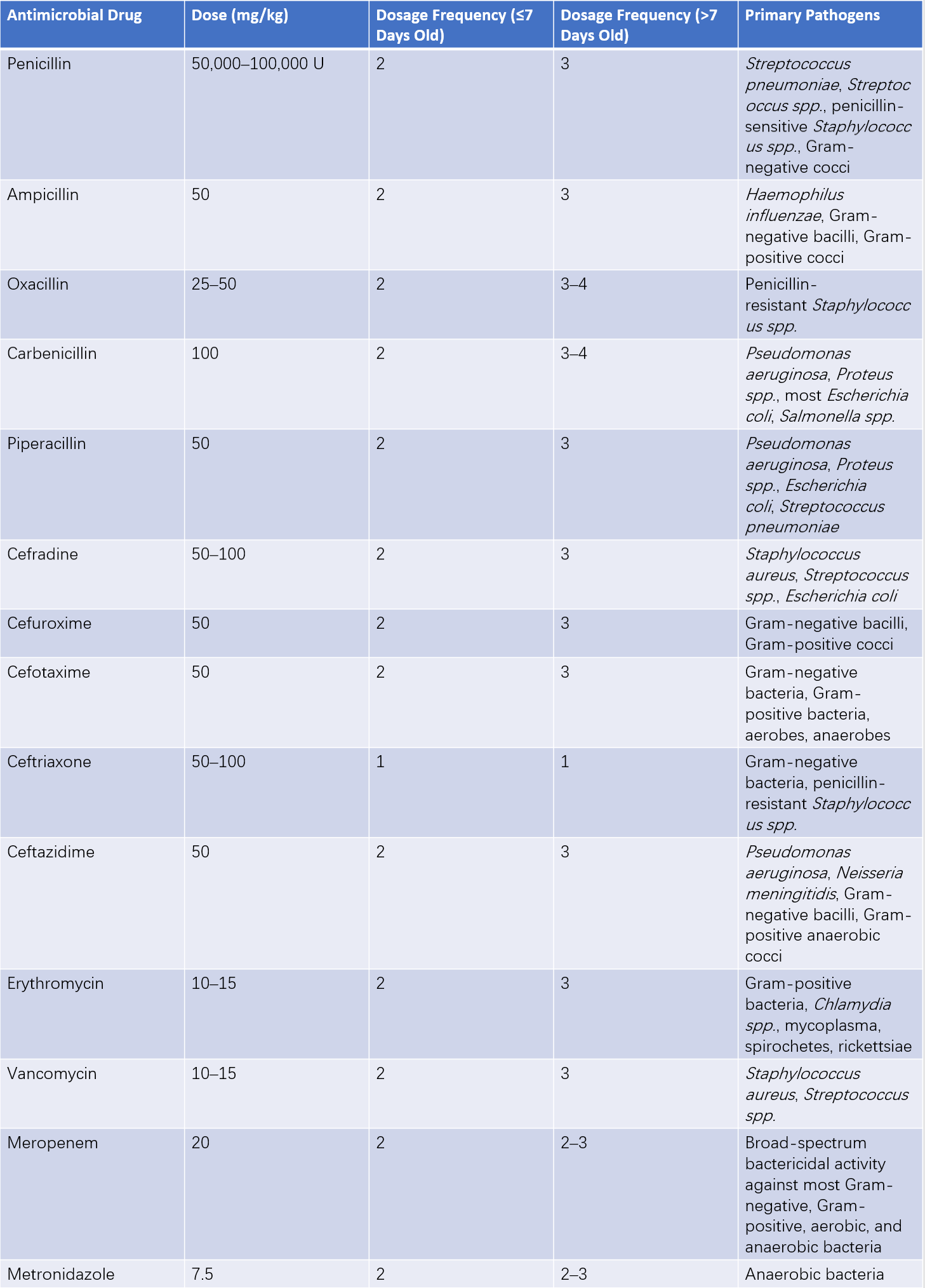
Table 1 Selection and usage guidelines for antimicrobial drugs in neonates
Adequate Duration
The treatment course is typically 10–14 days; for cases with complications, treatment duration extends to 3–4 weeks.
Monitoring Drug Toxicity
Dosage frequency should be adjusted for neonates (especially preterm infants) under one week of age due to immature hepatic and renal function. Aminoglycosides, known for ototoxicity, are currently contraindicated in the neonatal period.
Management of Severe Complications
This involves:
- Shock management.
- Correction of acidosis and hypoxemia.
- Reduction of cerebral edema.
Supportive Therapy
Measures include maintaining adequate warmth, providing sufficient caloric and fluid intake, and ensuring blood glucose and electrolyte levels remain within normal ranges.
Immunotherapy
Intravenous Immunoglobulin (IVIG)
Administration at 400 mg/kg per day for 5 consecutive days may help increase IgG levels.
Exchange Transfusion
This may be considered for critically ill neonates, with a total exchange volume of 100–150 mL/kg.