Hemolytic disease of the newborn (HDN), also known as isoimmune hemolytic disease, refers to hemolysis caused by blood group incompatibility between the mother and infant. Among the 26 identified human blood group systems, ABO incompatibility is the most common, while Rh incompatibility is less frequently observed.
Etiology and Pathogenesis
Fetal red blood cell antigens, which are inherited from the father and not present in the mother, pass through the placenta and enter the maternal circulation, stimulating the mother to produce corresponding blood group antibodies. When incomplete antibodies (IgG) enter the fetal bloodstream, they bind to antigens on fetal red blood cells, resulting in the sensitization and destruction of red blood cells in the reticuloendothelial system, leading to hemolysis. If the fetal red blood cells do not enter the mother's circulation until childbirth, the antibodies produced in the mother may not affect the current fetus but could cause disease in subsequent pregnancies with the same blood group incompatibility.
ABO Hemolysis
ABO hemolysis primarily occurs when the mother has blood type O and the fetus has blood type A or B. Incompatibility does not occur if the mother has blood type AB or the infant has blood type O.
Approximately 40%–50% of ABO hemolytic disease occurs in the first pregnancy. This is because O blood type mothers may have been sensitized to A or B blood group substances (e.g., from certain plants, parasites, typhoid vaccines, tetanus toxoid, or diphtheria toxoid) before pregnancy, resulting in the production of anti-A or anti-B antibodies (IgG).
The occurrence rate of blood group incompatibility between mother and fetus is about 15% when the mother is type O and the infant is type A or B. However, ABO hemolytic disease occurs in only one-fifth of incompatibilities. Contributing factors include the following:
- Variations in fetal red blood cell antigenicity lead to low levels of antibody production, amounting to only one-fourth of the adult quantity.
- A and B antigens are present in other tissues besides red blood cells. Only a small portion of antibodies cross the placenta and bind to fetal red blood cells, while the majority are absorbed by soluble A or B substances in tissues or plasma.
Rh Hemolysis
The Rh blood group system consists of six antigens: D, E, C, c, d, and e (the "d" antigen has not been identified but is inferred). The antigenicity of these antigens follows this order: D > E > C > c > e. Rh hemolysis most commonly involves RhD, followed by RhE, while Rhe hemolysis is rare due to the weak antigenicity of the "e" antigen. Traditionally, the absence of the D antigen on red blood cells is referred to as Rh-negative, while the presence of the antigen is termed Rh-positive.
If the mother is Rh-negative (lacking the D antigen) and the fetus inherits other Rh antigens such as E, hemolytic disease due to Rh incompatibility can occur.
Maternal exposure to Rh-incompatible antigens can commonly result from the following:
- Receipt of Rh-incompatible blood transfusions.
- Contact with Rh antigens during delivery or miscarriage, with exposure rates reaching 50%.
- Fetal Rh-positive red blood cells passing through the placenta into the maternal circulation during pregnancy.
Rh hemolytic disease is usually absent in the first pregnancy because Rh antibodies are not found in nature and must be produced in response to exposure to Rh antigens from human red blood cells. During the first pregnancy, Rh-negative mothers may be exposed to Rh-positive fetal red blood cells late in pregnancy or during placental separation (including miscarriage or curettage), initiating a primary immune response with IgM antibody production within approximately 8–9 weeks. IgM antibodies, however, do not cross the placenta, and only small quantities of IgG antibodies are subsequently produced, typically after delivery. In subsequent pregnancies, fetal red blood cells from an Rh-incompatible fetus can enter the maternal circulation in small amounts (as little as 0.2 mL), eliciting a secondary immune response. This response results in the rapid production of large quantities of IgG antibodies, which can cross the placenta and cause hemolysis in the fetus.
Rh-negative mothers who have previously received Rh-positive blood transfusions may also have affected first pregnancies.
Despite the strong antigenicity of RhD, only 1 in 20 RhD-incompatible pregnancies leads to disease, likely due to variations in maternal sensitivity to fetal Rh antigens. Additionally, if the father is heterozygous for the RhD allele, there is a 50% chance of the fetus inheriting RhD positivity, whereas with homozygous fathers, the likelihood is 100%. For other Rh antigens, similar inheritance patterns apply. ABO incompatibility can reduce the likelihood of Rh hemolysis because ABO antibodies may destroy fetal red blood cells entering the maternal circulation, preventing Rh antigens from triggering an immune response.
Pathophysiology
ABO hemolysis primarily causes jaundice, with other changes being relatively minor. In contrast, Rh hemolysis can lead to severe fetal anemia and even heart failure. Severe anemia, along with hypoalbuminemia and heart failure, can result in generalized edema (hydrops fetalis). Enhanced extramedullary hematopoiesis due to anemia can lead to hepatosplenomegaly. Bilirubin produced by red blood cell breakdown in the fetus is metabolized by the maternal liver via the placenta, resulting in minimal jaundice at birth. However, after delivery, the limited ability of neonates to process bilirubin leads to the appearance of jaundice. Excessively high levels of unconjugated bilirubin in the serum can result in bilirubin encephalopathy.
Clinical Manifestations
The severity of symptoms generally correlates with the degree of hemolysis. Most infants with ABO hemolytic disease exhibit jaundice with no other obvious abnormalities, while Rh hemolytic disease tends to be more severe, with extreme cases resulting in fetal death.
Jaundice
Most infants with Rh hemolytic disease develop jaundice within the first 24 hours after birth, with a rapid progression. For ABO hemolytic disease, jaundice usually appears within 24 hours after birth, with a marked increase on the second or third day of life. Serum bilirubin is predominantly unconjugated; however, severe hemolysis may lead to cholestasis, causing a subsequent rise in conjugated bilirubin levels.
Anemia
The severity of anemia varies. Severe Rh hemolysis may result in significant anemia or even heart failure immediately after birth. Some infants may develop late anemia between 3 and 6 weeks postnatally due to the persistence of maternal antibodies.
Hepatosplenomegaly
Hepatosplenomegaly of varying degrees is commonly seen in infants with Rh hemolytic disease, while it is less pronounced in those with ABO hemolytic disease.
Complications
Bilirubin Encephalopathy
Bilirubin encephalopathy represents the most serious complication of neonatal hemolytic disease. It primarily occurs in neonates with a total serum bilirubin (TSB) level exceeding 20 mg/dL (342 μmol/L) and/or a rise in bilirubin greater than 0.5 mg/dL per hour (8.5 μmol/L per hour), typically in infants born after 35 weeks of gestation. In low birth weight infants, bilirubin encephalopathy may develop at lower TSB levels, often ranging from 10–14 mg/dL (171–239 μmol/L). Symptoms usually emerge between the fourth and seventh days after birth.
Excessive levels of unconjugated bilirubin can cross the blood-brain barrier in its free form, causing central nervous system dysfunction. Without timely intervention, this may lead to permanent damage. Bilirubin commonly causes necrosis in the basal ganglia, hippocampus, hypothalamic nuclei, and cerebellar neurons. On autopsy, yellow staining of the affected neural nuclei may be observed, giving rise to the term "kernicterus."
The terms "bilirubin encephalopathy" and "kernicterus" are often used interchangeably in clinical settings. However, the recommended classification distinguishes between acute bilirubin encephalopathy (ABE), which describes central nervous system damage caused by bilirubin within the first weeks after birth, and kernicterus or chronic bilirubin encephalopathy, which denotes the long-term, irreversible sequelae of such damage.
Elevated bilirubin levels may also lead to transient encephalopathy, which refers to reversible neurological damage caused by bilirubin. Symptoms emerge as bilirubin levels increase, including lethargy and poor responsiveness. These symptoms resolve with treatment and subsequent reduction in bilirubin levels. Brainstem auditory evoked potentials (BAEP) may show prolonged wave latencies, which can return to normal following treatment.
Bilirubin encephalopathy often progresses rapidly within 24 hours and is clinically divided into four stages:
- Stage 1: Symptoms include lethargy, poor responsiveness, weak sucking, diminished Moro reflex, hypotonia, occasional high-pitched cries, and vomiting. This stage lasts approximately 12–24 hours.
- Stage 2: Convulsions, opisthotonos, and fever commonly occur, often coinciding with seizures. Mild cases present with a fixed gaze, while severe cases exhibit hypertonia, apnea, tightly clenched fists, extended and internally rotated arms, and opisthotonos. This stage lasts approximately 12–48 hours.
- Stage 3: During this stage, feeding and responsiveness improve, seizure frequency decreases, opisthotonos subsides, and muscle tone gradually normalizes. This stage lasts approximately 2 weeks.
- Stage 4: Characterized by the development of kernicterus sequelae, including:
- Athetosis: Involuntary, purposeless, and uncoordinated movements.
- Ocular Motility Disorders: Impaired upward gaze, leading to the "sunset eye" phenomenon.
- Hearing Impairment: Sensorineural hearing loss, particularly at high frequencies.
- Enamel Hypoplasia: Green or dark brown discoloration of the teeth.
Additional long-term effects may include cerebral palsy, intellectual disability, seizures, poor head control, and excessive drooling.
Neurological Dysfunction Induced by Bilirubin
Apart from typical bilirubin encephalopathy, some neonates exhibit subtle neurodevelopmental impairments without the classic clinical manifestations of bilirubin encephalopathy or kernicterus. This condition is referred to as bilirubin-induced neurological dysfunction (BIND) or subtle kernicterus. Clinical presentations may include mild cognitive abnormalities, isolated hearing deficits, or auditory neuropathy spectrum disorder (ANS).
Laboratory Tests
Maternal and Neonatal Blood Group Testing
Testing of maternal and neonatal ABO and Rh blood groups confirms the existence of blood group incompatibility.
Evaluation of Hemolysis
Indicators of Hemolysis
Reduction in red blood cell count and hemoglobin levels can occur with hemolysis. Early neonatal anemia is diagnosed when hemoglobin levels are below 140 g/L. Reticulocyte count may be elevated (>6%), peripheral blood smear may show an increased presence of nucleated red blood cells (>10 per 100 white blood cells) and spherocytes, while serum total bilirubin and unconjugated bilirubin are markedly increased.
End-Tidal Carbon Monoxide (ETCO) Measurement
During heme degradation, carbon monoxide (CO) is released. Measuring the CO concentration in exhaled air reflects the rate of red blood cell destruction or bilirubin production. This measurement can help predict the risk of severe hyperbilirubinemia in infants with hemolytic disease. If ETCO measurement is unavailable, blood levels of carboxyhemoglobin (COHb) may serve as an alternative indicator of hemolysis or bilirubin production.
Assessment of Sensitized Red Blood Cells and Blood Group Antibodies
Modified Direct Antiglobulin Test (DAT)
This test, also referred to as the modified Coombs test, involves mixing optimally diluted anti-human globulin serum with a saline suspension of washed test red cells. A positive result, indicated by red cell agglutination, confirms the presence of sensitized red blood cells. DAT has a high positivity rate in Rh hemolytic disease but lower positivity in ABO hemolytic disease.
Antibody Elution Test
Also known as the elution test, this test involves releasing blood group antibodies from sensitized red blood cells in the infant's blood through heat elution. These antibodies are used to sensitize adult red cells of the same blood group (for ABO system) or type O standard red cells (for Rh system). The addition of anti-human globulin serum produces agglutination if the test is positive. Both Rh and ABO hemolytic diseases generally produce positive results. Although the elution test is highly sensitive, its positivity in patients with negative direct antiglobulin tests does not always correspond to clinically significant hemolysis. Comprehensive evaluation with other indicators is thus recommended.
Free Antibody Test
Adult red cells or type O standard red cells are added to the infant's serum and sensitized, followed by the addition of anti-human globulin serum. A positive result indicates the presence of free ABO or Rh blood group antibodies in the serum, which may bind to red cells and cause hemolysis. This test assists in estimating the likelihood of continued hemolysis or the effectiveness of exchange transfusion but does not serve as a definitive diagnostic test.
Diagnosis
Prenatal Diagnosis
Pregnant women with a history of unexplained fetal loss, miscarriage, or neonatal severe jaundice, along with their partners, should undergo ABO and Rh blood group testing. In cases of incompatibility, antibody screening in maternal serum is recommended. The maternal level of IgG anti-A or anti-B antibodies does not significantly predict the likelihood of ABO hemolytic disease. Rh-negative pregnant women should have their Rh blood group antibody levels measured at 16 weeks of gestation as a baseline. Monitoring every 2–4 weeks thereafter can detect rising antibody titers, which indicate a potential risk for Rh hemolytic disease.
Postnatal Diagnosis
Diagnosis of Hemolysis
Hemolysis is suspected when jaundice appears early and progressively worsens after birth in the presence of maternal-neonatal blood group incompatibility. A positive modified Coombs test and antibody elution test confirm the diagnosis. Additional tests to aid in diagnosing hemolysis include peripheral blood smear (to detect spherocytes, reticulocytes, and nucleated red blood cells) and measurements of end-tidal carbon monoxide (ETCO) or blood carboxyhemoglobin (COHb) levels.
Auxiliary Diagnosis of Bilirubin Encephalopathy
Cranial MRI Scan
Bilirubin's neurotoxic effects typically involve selective brain regions, most commonly the globus pallidus of the basal ganglia. Cranial MRI has significant diagnostic value for bilirubin encephalopathy. During the acute phase, symmetrical T1-weighted hyperintensity in the globus pallidus is the hallmark finding. However, this feature does not strongly correlate with long-term prognosis. Over weeks to months, the T1-weighted hyperintensity resolves, but the development of T2-weighted hyperintensity in the same region indicates chronic bilirubin encephalopathy (kernicterus) and suggests poor prognosis.
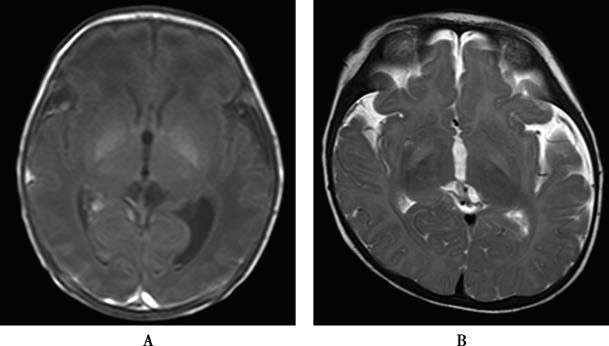
Figure 1 Bilirubin encephalopathy MRI scans
A. Symmetrical T1 hyperintensity in bilateral globus pallidus (Day 6 postpartum).
B. Symmetrical T2 hyperintensity in bilateral globus pallidus (Month 4 postpartum).
Brainstem Auditory Evoked Potentials (BAEP)
BAEP reflects the bioelectric responses originating from the cochlear nerve and auditory structures in the brainstem. It is often used to screen for auditory nerve damage caused by bilirubin encephalopathy. BAEP is a sensitive indicator of acute bilirubin neurotoxicity and may be the earliest or sole manifestation. Its noninvasive and objective nature makes it suitable for early diagnosis and monitoring of bilirubin encephalopathy progression. Central nervous system toxicity from elevated serum bilirubin can be assessed by observing the latency of BAEP waveforms I, III, and V and the interpeak latency of waves I–III and III–V. Changes in BAEP during the acute phase of bilirubin encephalopathy may resolve with timely treatment and decreases in serum bilirubin levels.
Differential Diagnosis
The condition requires differentiation from the following diseases:
Congenital Nephrotic Syndrome
This condition presents with generalized edema, hypoalbuminemia, and proteinuria but lacks pathological jaundice and hepatosplenomegaly.
Neonatal Anemia
Neonatal anemia caused by twin-to-twin transfusion syndrome or maternal-fetal transfusion does not involve severe jaundice, blood group incompatibility, or positive hemolysis tests.
Physiological Jaundice
ABO hemolytic disease may manifest solely as jaundice, making it easily confused with physiological jaundice. The presence of blood group incompatibility and positive hemolysis tests helps distinguish it.
Treatment
Prenatal Treatment
Early Delivery
In Rh-negative pregnant women with a history of transfusion, stillbirth, miscarriage, or delivery, a gradual increase in Rh antibody titer to 1:32 or 1:64 or higher, along with elevated amniotic fluid bilirubin levels measured by spectrophotometry and an L/S ratio >2 (indicating fetal lung maturity), suggests the feasibility of early delivery.
Plasma Exchange
For pregnant women with significantly elevated Rh antibody titers in whom early delivery is not appropriate, plasma exchange can be performed to remove antibodies and reduce fetal hemolysis. However, this treatment is rarely applied in clinical practice.
Intrauterine Blood Transfusion
For cases of fetal hydrops or fetal hemoglobin <80 g/L with immature lungs, concentrated red blood cells that do not agglutinate with maternal serum can be injected into the umbilical vein or fetal abdominal cavity under ultrasound guidance to correct anemia. In countries and regions where Rh immunoglobulin prophylaxis is widely administered, severe intrauterine hemolysis has become rare, and this technique is now rarely used.
Phenobarbital
Maternal oral administration of phenobarbital 1–2 weeks before the expected delivery date may induce fetal UGT (uridine diphosphate-glucuronosyltransferase) activity, reducing neonatal jaundice. However, the preventative efficacy of this method lacks data from clinical randomized controlled trials.
Neonatal Treatment
Phototherapy (Light Therapy)
Indications
When serum total bilirubin levels rise, phototherapy is indicated according to gestational age, the presence of high-risk factors, postnatal age, and reference to phototherapy treatment nomograms.
Mechanism
Phototherapy facilitates the photoisomerization of unconjugated bilirubin, forming configurational isomers such as 4Z,15E-bilirubin IX, ZE; 4E,15Z-bilirubin IX, EZ, and structural isomers like lumirubin (LR). These water-soluble isomers are excreted directly through bile and urine without requiring hepatic metabolism. Light wavelengths of 460–490 nm, with a peak at 475 nm (blue light), are most effective. Fluorescent or natural light has some effect but should avoid direct sunlight exposure to prevent skin damage from ultraviolet radiation. Because phototherapy primarily affects superficial tissue layers, the subsidence of skin jaundice does not necessarily indicate normalization of serum unconjugated bilirubin levels, necessitating monitoring of serum bilirubin.
Equipment
Phototherapy devices include phototherapy boxes, phototherapy lamps, LED lights, and phototherapy blankets. Techniques include single-sided and double-sided phototherapy. Factors influencing phototherapy efficacy include light source type and intensity, single or multiple light sources, distance between the light source and target, body surface area exposed, and treatment duration. Irradiance is calculated based on the amount of light received by the target's surface, measured in μW/(cm2·nm) using a radiometer. A higher irradiance correlates with a faster rate of bilirubin reduction. Standard phototherapy irradiance is 8–10 μW/(cm²·nm), while intensive phototherapy exceeds 30 μW/(cm2·nm). Intensive phototherapy is commonly recommended. During treatment, the infant's eyes are protected with black eye shields to prevent retinal damage, and areas like the perineum and anus are covered with a diaper, while the rest of the body remains exposed. Phototherapy can be continuous or applied intermittently, such as 12-hour sessions.
Side Effects
Phototherapy may cause fever, diarrhea, and rashes, although these are generally mild and resolve spontaneously after the therapy is paused. When serum conjugated bilirubin exceeds 68 μmol/L (4 mg/dL) and levels of alanine transaminase and alkaline phosphatase are elevated, the skin may take on a bronzed appearance, known as bronze baby syndrome, requiring cessation of phototherapy. This condition typically resolves spontaneously. Adequate hydration is also necessary during phototherapy.
Monitoring
Bilirubin levels are monitored closely during phototherapy, usually every 6–12 hours. In neonates older than 35 weeks gestation, phototherapy is typically discontinued once serum total bilirubin levels drop below 13–14 mg/dL (222–239 μmol/L).
Pharmacological Treatment
Albumin Administration
When serum bilirubin levels approach the threshold for exchange transfusion and blood albumin levels are below 25 g/L, plasma (10–20 mL/kg per dose) or albumin (1 g/kg per dose) can be administered. This increases bilirubin binding to albumin, reducing the risk of bilirubin encephalopathy.
Correction of Metabolic Acidosis
In cases of severe acidosis, sodium bicarbonate can be used to raise blood pH, promoting the binding of unconjugated bilirubin to albumin.
Liver Enzyme Inducers
Agents capable of inducing UGT (uridine diphosphate-glucuronosyltransferase) enzyme activity enhance the liver's ability to conjugate and excrete bilirubin. Phenobarbital (5 mg/kg per day, administered orally in 2–3 divided doses for 4–5 days) can be utilized for this purpose.
Intravenous Immunoglobulin (IVIG)
IVIG blocks Fc receptors in the mononuclear phagocyte system, thereby inhibiting phagocyte-mediated destruction of antibody-sensitized red blood cells. The typical dose is 0.5–1 g/kg, administered via intravenous infusion over 2–4 hours. Early application in cases of ABO or Rh blood group incompatibility with positive direct antiglobulin tests has shown effective clinical outcomes, with the possibility of repeated dosing if necessary.
Exchange Transfusion Therapy
Functions
This removes free antibodies and sensitized red blood cells, reducing hemolysis, eliminates significant amounts of bilirubin from the bloodstream, preventing bilirubin encephalopathy, and corrects anemia, improves oxygen-carrying capacity, and prevents heart failure.
Indications
Exchange transfusion is necessary for most cases of Rh hemolytic disease and select severe cases of ABO hemolytic disease. Any of the following conditions may warrant exchange transfusion:
- For preterm infants ≥35 weeks of gestational age and full-term infants, Figure 6-15 serves as a reference. If total serum bilirubin (TSB) levels do not decrease or continue to rise after 4–6 hours of intensive phototherapy, or if the decrease in TSB after phototherapy in immune-mediated hemolysis is less than 2–3 mg/dL (34–50 μmol/L), exchange transfusion is initiated promptly.
- Severe hemolysis presenting with an umbilical cord bilirubin level >4.5 mg/dL (76 μmol/L) and hemoglobin <110 g/L at birth, along with symptoms such as hydrops, hepatosplenomegaly, and heart failure.
- Clinical manifestations of acute bilirubin encephalopathy, irrespective of whether bilirubin levels meet the threshold for exchange transfusion, or if TSB levels significantly decrease during preparation for exchange transfusion.
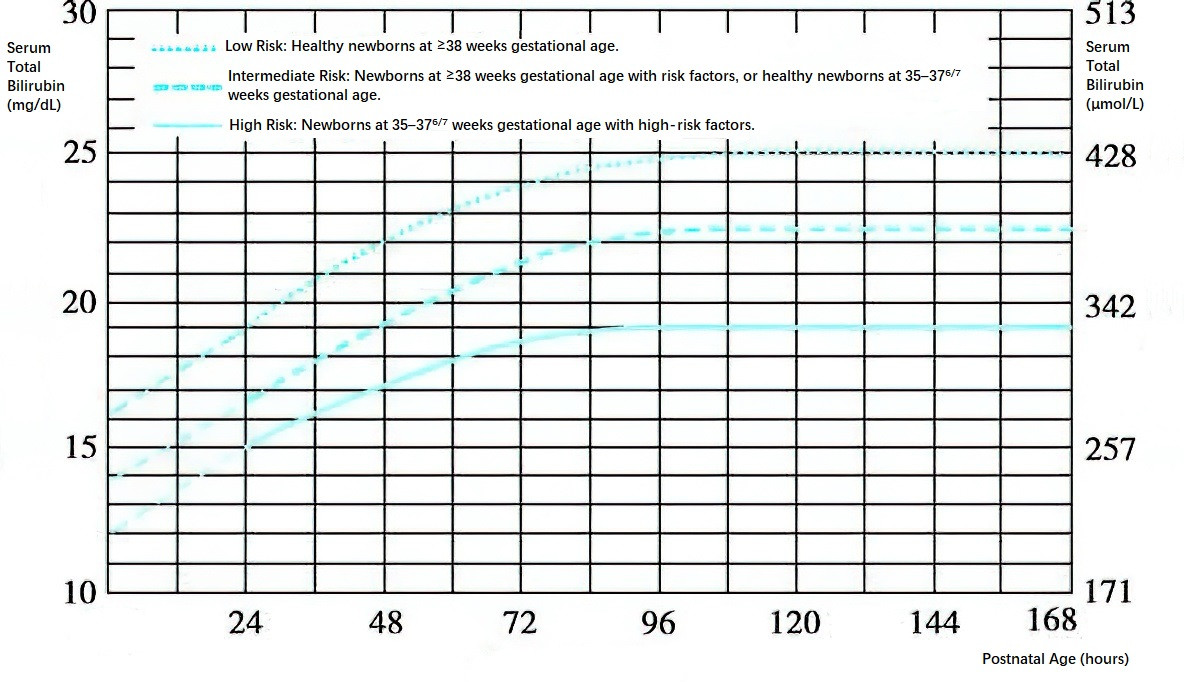
Figure 2 Exchange transfusion reference standards for preterm infants ≥35 weeks gestational age and full-term infants
Procedures
Blood Source
For Rh hemolytic disease, blood should match the Rh type of the mother and the ABO type of the infant. In emergencies or when no matching blood is available, type O blood can be used. In cases of ABO hemolytic disease involving a type O mother and type A or B infant, a mixture of AB plasma and type O red blood cells is preferred. For severe anemia with heart failure, packed red blood cells diluted with half the usual volume of plasma may be used.
Exchange Volume
Generally, twice the infant's blood volume (approximately 150–180 mL/kg) is exchanged, which removes about 85% of sensitized red blood cells and 60% of bilirubin and antibodies.
Route
The umbilical vein or other large veins are commonly used for exchange transfusion. In some cases, synchronous exchange via the umbilical artery and vein or via peripheral arteries and veins may be performed.
Additional Treatment
Measures to address complications include the prevention of hypoglycemia, hypocalcemia, and hypothermia, as well as correction of hypoxia, anemia, edema, electrolyte imbalances, and heart failure.
Prevention
Rh-negative women who experience miscarriage or deliver an Rh-positive first pregnancy are advised to receive appropriate Rh immunoglobulin as early as possible to neutralize Rh antigens that have entered the maternal circulation. The current clinical approach involves intramuscular administration of 300 μg of anti-D immunoglobulin to RhD-negative women at 28 weeks of gestation and again within 72 hours after delivering an RhD-positive fetus. This method provides a protection rate of up to 95% against hemolytic disease in the second pregnancy, significantly reducing the number of Rh hemolysis-associated cases requiring exchange transfusion in newborns in Western countries in recent years.