Neonatal jaundice, also referred to as neonatal hyperbilirubinemia, is a condition characterized by yellow discoloration of the skin or other organs due to bilirubin accumulation in the body. It is the most common clinical issue during the neonatal period, with over 80% of healthy newborns developing visible yellowing of the skin in the early days after birth. A total serum bilirubin (TSB) level of 5–7 mg/dL in neonates (compared to >2 mg/dL in adults) is sufficient to produce visible jaundice (bilirubin conversion factor: 1 mg/dL = 17.1 μmol/L). Unconjugated hyperbilirubinemia is the most common form of neonatal jaundice. In severe cases, it can lead to bilirubin encephalopathy (kernicterus), resulting in permanent neurological damage or even death.
Physiological Mechanisms of Bilirubin Metabolism in the Fetus and Neonate
During the fetal period, the liver exhibits relatively low metabolic activity. Bilirubin produced from red blood cell breakdown in the fetus is primarily transferred through the placenta to the maternal circulation for processing by the maternal liver. When excessive red blood cell destruction occurs due to hemolysis in utero, the maternal liver may fail to process all bilirubin produced by the fetus, leading to elevated bilirubin levels in the umbilical cord blood of the neonate. In cases of intrauterine hemolysis, yellow discoloration of the umbilical cord tissue and amniotic fluid, along with fetal anemia (if bone marrow and extramedullary hematopoiesis cannot meet demands), may also occur.
Bilirubin originates from the breakdown of heme-containing proteins, with the majority in neonates derived from the destruction of senescent red blood cells. Approximately 80% of heme degradation and bilirubin production occurs through the reticuloendothelial system, while the remaining 20% comes from heme in liver, bone marrow erythroid precursors, and other tissues. Heme is converted to biliverdin by heme oxygenase-1 (HO-1) and subsequently to bilirubin by biliverdin reductase. Endogenous carbon monoxide (CO) is produced during the conversion of heme to biliverdin. Clinically, the rate of red blood cell breakdown or bilirubin production can be estimated by measuring the exhaled CO. Each gram of hemoglobin generates approximately 34 mg (600 μmol) of unconjugated bilirubin.
Bilirubin Transport, Hepatic Uptake, and Metabolism
In the bloodstream, most unconjugated bilirubin binds to albumin and is transported to the liver as a bilirubin-albumin complex. Bound bilirubin typically does not penetrate the central nervous system; however, unbound ("free") unconjugated bilirubin, being lipophilic, can cross the blood-brain barrier and enter the central nervous system. Excessive free bilirubin levels can lead to bilirubin encephalopathy. Several factors can significantly reduce bilirubin-albumin binding capacity, including low serum albumin levels, a rapid rise in bilirubin concentration, hemolysis, asphyxia, hypoxia, acidosis, infection, prematurity, and hypoglycemia. Additionally, substances such as free fatty acids, intravenous lipid emulsions, and certain drugs (e.g., sulfonamides, cephalosporins, and diuretics) may compete with bilirubin for albumin binding, thereby increasing free bilirubin levels.
After entering hepatocytes, bilirubin binds to receptor proteins, such as Y protein and Z protein (intracellular transport proteins), before being transported to the smooth endoplasmic reticulum. There, it is conjugated with two molecules of glucuronic acid via the catalytic action of UDP-glucuronosyltransferase (UGT), forming water-soluble conjugated bilirubin. Conjugated bilirubin is excreted into bile and reaches the intestines, where bacterial enzymes convert it into stercobilinogen, which is then excreted in feces. Some of the conjugated bilirubin in the gut can be hydrolyzed by β-glucuronidase or directly deconjugated in an alkaline environment to revert to unconjugated bilirubin. The unconjugated bilirubin may then be reabsorbed through the intestinal wall into the portal circulation, reenter the liver, and undergo enterohepatic circulation. In certain conditions, such as prematurity or intestinal obstruction, enterohepatic circulation may significantly increase blood bilirubin levels.
Characteristics of Neonatal Bilirubin Metabolism
Several factors contribute to the higher serum bilirubin levels commonly observed during the neonatal period:
Excessive Bilirubin Production
Newborns produce considerably more bilirubin daily than adults (6–10 mg/kg vs. 3–4 mg/kg), largely due to increased red blood cell turnover. Fetal hypoxemia leads to a compensatory increase in red blood cells, which are subsequently destroyed after birth as oxygen saturation improves. Neonatal red blood cells have a shorter lifespan (less than 70 days in preterm infants, about 80 days in term infants, compared to 120 days in adults), and their hemoglobin turnover rate is double that of adults. The proportion of heme derived from liver and other tissues, as well as from bone marrow erythroid precursors, is higher in neonates (20%–25% in term infants and 30% in preterm infants) compared to adults (15%).
Reduced Capacity of Plasma Albumin to Bind Bilirubin
Newborns commonly experience varying degrees of acidosis, which reduces the binding of free bilirubin to albumin. Preterm infants, particularly those with lower gestational age, have lower albumin levels, further limiting their bilirubin-binding capacity.
Impaired Hepatic Bilirubin Metabolism
After entering hepatocytes, unconjugated bilirubin binds to Y and Z proteins; however, the levels of Y protein are very low at birth (normalizing by days 5–10). UGT levels are also low (approaching normal within the first week) and have suboptimal activity (only 0%–30% of normal), resulting in reduced conjugated bilirubin production. The ability of hepatocytes to excrete conjugated bilirubin into the intestines is also temporarily reduced at birth, particularly in preterm infants, leading to transient intrahepatic cholestasis.
Enterohepatic Circulation
In neonates, poor intestinal motility and an immature gut microbiota contribute to increased enterohepatic circulation. The activity of β-glucuronidase in the gut is relatively high, converting conjugated bilirubin back to unconjugated bilirubin, which is then reabsorbed. Increased reabsorption is especially evident under conditions such as delayed meconium passage or constipation.
Additional factors, such as starvation, hypoxia, dehydration, acidosis, hemangiomas of the skin or internal organs, and internal hemorrhage (e.g., cephalohematomas or intracranial bleeding), may predispose neonates to jaundice or exacerbate existing jaundice.
Classification of Neonatal Jaundice
The traditional view of neonatal jaundice, which categorizes it as either "physiological" or "pathological" based on a single serum bilirubin value, has been challenged. A more widely accepted approach for assessing the risk of hyperbilirubinemia involves using age-specific (in days or hours) bilirubin nomograms, such as the Bhutani curve. This method evaluates bilirubin levels based on gestational age, hours since birth, and the presence of risk factors to determine whether these levels are normal or safe and whether therapeutic interventions (such as phototherapy) are required. Risk factors refer to clinical conditions commonly associated with severe hyperbilirubinemia, which increase not only the likelihood of significant hyperbilirubinemia but also the risk of bilirubin-induced neurological dysfunction. Hemolysis in neonates, cephalohematomas, organ or subcutaneous hematomas, asphyxia, hypoxia, acidosis, sepsis, hyperthermia, hypothermia, hypoalbuminemia, and hypoglycemia are examples of risk factors.
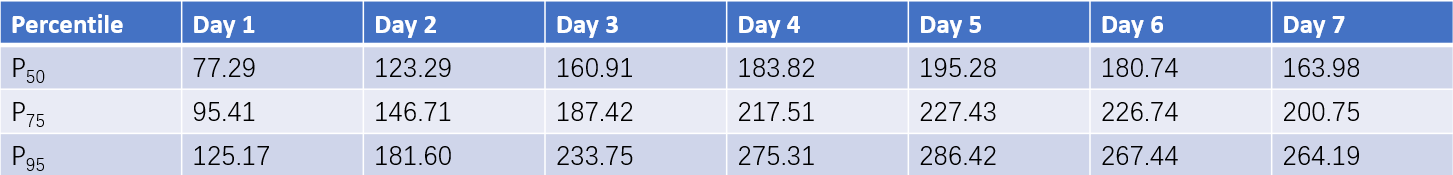
Table 1 Percentile values of bilirubin levels within the first 7 days in 875 full-term neonates (Unit: μmol/L)
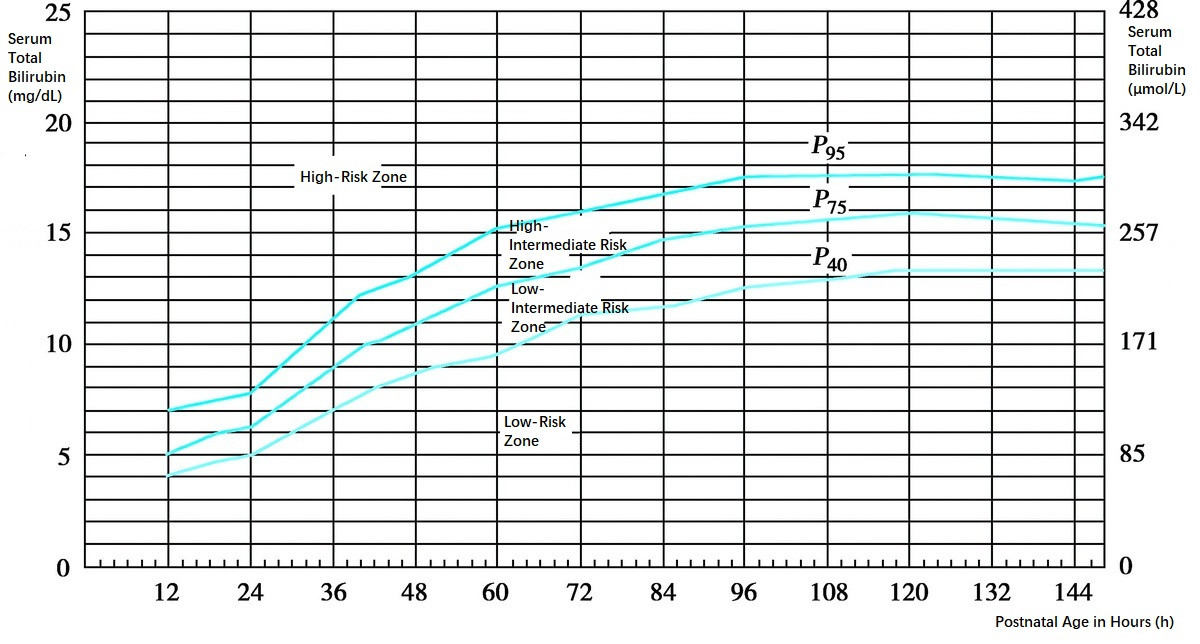
Figure 1 Hour-specific Bilirubin risk nomogram (Bhutani curve)
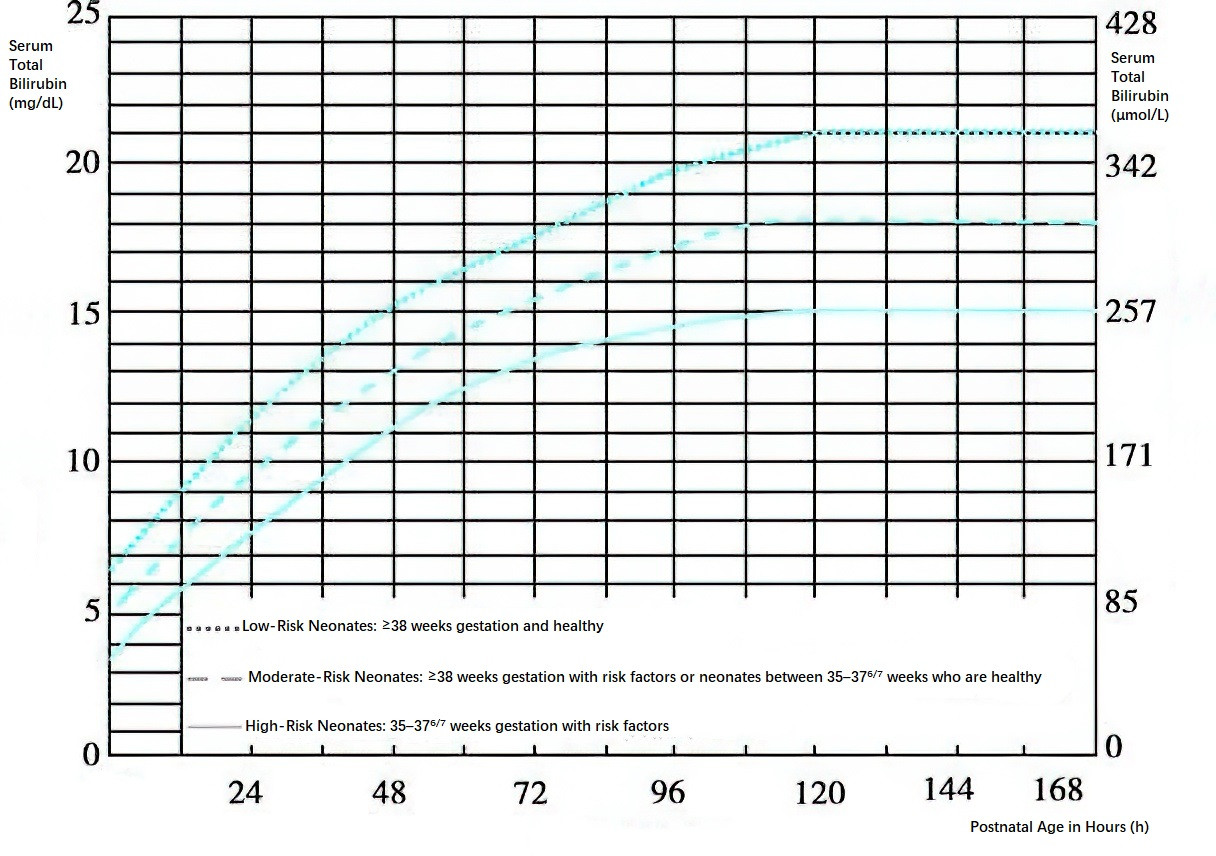
Figure 2 Hour-specific phototherapy intervention thresholds for neonates above 35 weeks of gestation
Physiological Jaundice
Also known as non-pathological hyperbilirubinemia, physiological jaundice arises primarily due to increased red blood cell breakdown and immaturity of the hepatic enzyme system at birth, resulting in bilirubin production exceeding the capacity for bilirubin excretion. Nearly all term neonates experience transient elevations in serum bilirubin levels during the first days of life to varying degrees. Table 6-8 outlines total serum bilirubin concentrations in term neonates from days 1 to 7 after birth. Physiological jaundice is an exclusionary diagnosis with the following characteristics:
- General health remains good.
- In term neonates, jaundice emerges between days 2–3 after birth, peaks around days 4–5, and fades by days 5–7, not persisting beyond two weeks. In preterm neonates, it typically develops between days 3–5, peaks by days 5–7, resolves by days 7–9, and may last up to 3–4 weeks in some cases.
- The daily increase in serum bilirubin levels is <85 μmol/L (5 mg/dL), or an hourly increase of <0.3 mg/dL during the first 24 hours of life or <0.2 mg/dL after 24 hours of life.
- Total serum bilirubin levels do not exceed the 95th percentile of the bilirubin nomogram (Bhutani curve) or fall below the threshold for phototherapy intervention, considering the neonate's postnatal age, gestational age, and associated risk factors.
Pathological Jaundice
Pathological jaundice, also referred to as non-physiological hyperbilirubinemia or significant hyperbilirubinemia, is distinguished by abnormally high serum bilirubin levels or altered patterns of bilirubin elevation compared to physiological jaundice. While some cases of significant bilirubin elevation may represent extensions or intensifications of physiological jaundice, it is essential to actively investigate and identify any underlying causes to intervene promptly and prevent bilirubin-induced neurological damage. The presence of any of the following features suggests pathological jaundice:
- Jaundice presenting within the first 24 hours after birth.
- Serum total bilirubin levels surpassing the age-specific phototherapy threshold or exceeding the 95th percentile on the bilirubin nomogram. Alternatively, a daily bilirubin rise of >85 μmol/L (5 mg/dL) or an hourly increase of >0.3 mg/dL within 24 hours of birth, or >0.2 mg/dL per hour thereafter.
- Prolonged jaundice, lasting >2 weeks in term neonates or >4 weeks in preterm neonates.
- Recurrent jaundice following initial resolution.
- Serum conjugated bilirubin levels exceeding 34 μmol/L (2 mg/dL).
Pathological jaundice can be classified into three types based on causative mechanisms:
Excessive Bilirubin Production
Elevated bilirubin levels may result from excessive red blood cell destruction or enhanced enterohepatic circulation.
- Polycythemia: Defined as venous hematocrit >65%, red blood cells >6×10¹²/L, or hemoglobin >220 g/L. Common causes include fetomaternal or twin-to-twin transfusion, delayed clamping of the umbilical cord, intrauterine growth restriction (secondary to chronic hypoxia), and neonates born to diabetic mothers.
- Extravascular Hemolysis: Conditions such as large cephalohematomas, subcutaneous hematomas, intracranial hemorrhage, or visceral bleeding.
- Alloimmune Hemolysis: Often occurs due to maternal-fetal blood type incompatibility, most commonly ABO or Rh incompatibility, with ABO hemolytic disease being more frequent.
- Infections: Severe infections caused by bacteria, viruses, spirochetes, chlamydia, mycoplasma, and protozoa can induce hemolysis, with sepsis resulting from Staphylococcus aureus or Escherichia coli being particularly common.
- Enhanced Enterohepatic Circulation: Conditions such as congenital intestinal atresia, congenital pyloric hypertrophy, Hirschsprung's disease, starvation, or delayed feeding can result in delayed meconium passage or constipation, leading to increased bilirubin reabsorption.
- Breastfeeding and Jaundice:
- Breastfeeding-Associated Jaundice: This often occurs in neonates during the first week of life and is typically related to insufficient milk intake, delayed stooling, and subsequent increases in serum bilirubin levels. It may also be referred to as suboptimal intake hyperbilirubinemia. Neonates may present with significant physiological weight loss and hypernatremia. Enhancing breastfeeding frequency and milk volume often alleviates jaundice, with formula supplementation used if needed. This type of jaundice does not contraindicate breastfeeding.
- Breast Milk Jaundice: This refers to jaundice persisting beyond 1–3 months in breastfed neonates, characterized by non-hemolytic hyperbilirubinemia. Its diagnosis is often exclusionary. Elevated levels of β-glucuronidase in maternal breast milk may increase intestinal deconjugation of bilirubin, enhancing its reabsorption and liver burden. Genetic polymorphisms in hepatic UGT may also contribute. Breast milk jaundice generally does not require treatment and often resolves after a brief interruption of breastfeeding (24–48 hours). However, breastfeeding can typically continue, and phototherapy is indicated if bilirubin levels reach treatment thresholds.
- Red Blood Cell Enzyme Deficiencies: Deficiencies in glucose-6-phosphate dehydrogenase (G-6-PD), pyruvate kinase, or hexokinase can impair red blood cell metabolism, leading to rigid cell membranes and increased destruction within the reticuloendothelial system.
- Red Blood Cell Membrane Abnormalities: Disorders such as hereditary spherocytosis, hereditary elliptocytosis, hereditary stomatocytosis, or infantile pyknocytosis result in structural abnormalities, increasing red blood cell destruction within the spleen.
- Hemoglobinopathies: Conditions such as thalassemia or abnormalities in hemoglobin chain quantity/quality may lead to hemolysis.
- Other Factors: Deficiencies in vitamin E or zinc can disrupt membrane integrity, resulting in hemolysis.
Bilirubin Metabolism Dysfunction in the Liver
Elevations in serum unconjugated bilirubin may occur due to impaired hepatic uptake and conjugation of bilirubin.
- Hypoxia and Infection: Conditions such as asphyxia and severe infections can inhibit the activity of UDP-glucuronosyltransferase (UGT) in the liver.
- Crigler-Najjar Syndrome: This congenital deficiency of UGT results from mutations in the coding region of the UGT1A1 gene.
- Type I follows an autosomal recessive inheritance pattern and is characterized by complete enzyme deficiency. UGT inducers, such as phenobarbital, are ineffective in this type. These patients require long-term phototherapy within the first years of life to reduce serum bilirubin levels and prevent bilirubin encephalopathy. This rare condition has a high mortality rate, and liver transplantation is necessary to restore sufficient UGT activity.
- Type II is generally autosomal dominant with partial UGT activity retained. It is more common than Type I, and treatment with enzyme inducers, such as phenobarbital, is effective.
- Gilbert Syndrome: A chronic, benign form of unconjugated hyperbilirubinemia that follows an autosomal dominant inheritance pattern. It results from impaired hepatic uptake of bilirubin and reduced UGT activity. Unlike Crigler-Najjar syndrome, the reduction in UGT activity is linked to increased repeats in the TA promoter region of the UGT1A1 gene or, more commonly, mutations in exon G71R. Symptoms are mild and often manifest during adolescence. In the neonatal period, reduced UGT activity causes impaired hepatic conjugation of bilirubin, leading to hyperbilirubinemia. Coexisting UGT mutations with other factors, such as G-6-PD deficiency or ABO blood type incompatibility, may exacerbate the hyperbilirubinemia.
- Lucey-Driscoll Syndrome: Also known as familial transient neonatal hyperbilirubinemia. Certain neonates present with severe unconjugated hyperbilirubinemia within the first 48 hours of life due to the presence of an unidentified UGT inhibitor in maternal serum during late pregnancy. This condition is often familial, with pronounced jaundice in the early neonatal period that resolves spontaneously at 2–3 weeks of life.
- Medications: Certain medications, such as sulfonamides, salicylates, vitamin K3, indomethacin, and digoxin, can compete with bilirubin for binding to Y and Z proteins, disrupting bilirubin transport and exacerbating jaundice.
- Congenital Hypothyroidism: In hypothyroidism, the reduced UGT activity can persist for weeks to months. Hepatic uptake and transport of bilirubin are also affected. Jaundice tends to resolve significantly with thyroid hormone therapy.
- Other Conditions: Elevated serum bilirubin levels or delayed resolution of physiological jaundice may be associated with pituitary hypofunction or trisomy 21 (Down syndrome).
Cholestasis and Impaired Bile Excretion
Impaired excretion of conjugated bilirubin by hepatocytes or obstruction of the biliary tract may result in conjugated hyperbilirubinemia. Liver dysfunction may also cause elevations in unconjugated bilirubin levels in such cases.
- Neonatal Hepatitis: Often caused by intrauterine infections due to viruses such as hepatitis B, cytomegalovirus, rubella virus, herpes simplex virus, enterovirus, and Epstein-Barr virus.
- Congenital Metabolic Disorders: Disorders such as α1-antitrypsin deficiency, galactosemia, fructose intolerance, tyrosinemia, glycogen storage disease type IV, and lipid storage diseases (e.g., Niemann-Pick disease and Gaucher disease) may cause hepatocyte damage.
- Dubin-Johnson Syndrome: A rare condition involving congenital nonhemolytic conjugated hyperbilirubinemia caused by impaired secretion and excretion of conjugated bilirubin by hepatocytes. Both conjugated and unconjugated bilirubin levels may be elevated. The clinical course is typically benign.
- Cholestasis Induced by Parenteral Nutrition: Prolonged total parenteral nutrition, particularly in extremely low birth weight infants in neonatal intensive care units, may lead to cholestasis. This condition involves elevated serum conjugated bilirubin levels and may be accompanied by liver dysfunction. Cholestasis generally resolves after the cessation of parenteral nutrition.
- Biliary Atresia and Other Causes of Bile Duct Obstruction: Congenital biliary atresia or congenital choledochal cysts are common causes of obstructive jaundice in the neonatal period, resulting from impaired bile flow due to intrahepatic or extrahepatic bile duct obstruction. Neonatal biliary atresia typically presents with jaundice at 2–4 weeks of life, pale or clay-colored stools, and significantly elevated serum conjugated bilirubin levels. Bile plug syndrome can also present with obstructive jaundice due to bile accumulation in small bile ducts. Severe neonatal hemolytic disease may result in transient bile stasis due to the need to excrete large amounts of bilirubin via the bile ducts. Additionally, tumors in the liver and biliary tract may compress the bile ducts and cause obstruction. For neonatal biliary atresia, early diagnosis and intervention are critical. Surgical drainage performed within the first 60 days of life yields better outcomes; otherwise, progressive biliary cirrhosis may lead to irreversible liver damage. Liver transplantation remains the treatment option for cases where surgical drainage is ineffective.
Risk Assessment and Management of Hyperbilirubinemia
Jaundice is a common phenomenon in the early neonatal period. While moderate physiological bilirubin levels provide beneficial antioxidant effects to the body, excessive levels of bilirubin can result in permanent neurological damage and dysfunction. During diagnosis and treatment, it is essential to identify hyperbilirubinemia with associated risks and treat it promptly while avoiding excessive or unnecessary intervention for physiological jaundice that has not reached a risk threshold. Neurological damage caused by neonatal hyperbilirubinemia can be entirely prevented through early intervention, such as phototherapy, at risky bilirubin levels. Unfortunately, instances of bilirubin encephalopathy in neonates still occur, highlighting the importance of risk assessment and systematic management of bilirubin levels in the early neonatal stage.
Peak bilirubin levels in neonatal hyperbilirubinemia typically occur around the 4th to 5th day after birth. By this time, most healthy, full-term neonates have already been discharged from maternity departments. A systematic measurement and follow-up of bilirubin levels in all neonates are therefore considered essential before and after hospital discharge.
Currently, the commonly used method for risk assessment involves the hour-specific bilirubin risk nomogram (Bhutani curve), which stratifies bilirubin levels for the corresponding postnatal age into four risk zones: low-risk, low-intermediate risk, high-intermediate risk, and high-risk, based on the percentiles P40, P75, and P95. This assessment is further combined with the evaluation of high-risk factors, such as neonatal hemolysis, cephalhematomas, subcutaneous hematomas, asphyxia, hypoxia, acidosis, sepsis, hyperthermia, hypothermia, hypoalbuminemia, or hypoglycemia. Intervention is generally recommended for neonates with total serum bilirubin levels exceeding the P95 threshold.
Using the hour-specific bilirubin risk nomogram, serum or transcutaneous bilirubin measurements should be performed to follow up on neonates discharged from maternity care.
For neonates discharged within 48 hours of birth, two follow-up visits are generally recommended, the first between 24–72 hours after birth and the second between 72–120 hours.
For neonates discharged between 72–120 hours of birth, follow-up is typically recommended within 2–5 days after discharge.
For neonates with risk factors, additional follow-up visits are considered necessary. For neonates without risk factors, longer intervals between follow-ups may be deemed acceptable.
The bilirubin level and risk zone prior to discharge should guide the formulation of an appropriate follow-up plan.
Treatment of Hyperbilirubinemia
Details are available in the section on hemolytic disease of the newborn.