Persistent pulmonary hypertension of the newborn (PPHN) is characterized by persistently elevated pulmonary vascular resistance after birth, which hinders the transition from fetal circulation to the normal "adult" circulation. This leads to significantly increased pressure in the pulmonary arteries and the right heart system, causing right-to-left blood shunting at the atrial and/or ductal level. Clinically, it manifests as severe hypoxemic respiratory failure. PPHN occurs in approximately 2 per 1,000 live births, but among all newborns with respiratory failure, the proportion with varying degrees of pulmonary hypertension can reach 10%. PPHN is more commonly observed in term or post-term infants but can also occur in preterm or extremely preterm infants. Most cases are associated with underlying pulmonary parenchymal disease, although idiopathic pulmonary vascular disorders can also be causative.
Etiology and Pathogenesis
Perinatal Asphyxia or Pulmonary Parenchymal Disease
PPHN often occurs secondary to pulmonary parenchymal diseases, with or without associated asphyxia, such as meconium aspiration syndrome, respiratory distress syndrome (RDS), severe transient tachypnea, pneumonia, or sepsis. These factors impair the adaptation of neonatal pulmonary vasculature to the postnatal environment, preventing effective vasodilation and maintaining a state of high vascular resistance, resulting in persistently elevated pulmonary arterial pressure.
Pulmonary Vascular Remodeling and Structural Abnormalities Caused by Chronic Intrauterine Hypoxia or Other Factors
PPHN caused by this mechanism may present with normal pulmonary parenchyma but with abnormal pulmonary vascular development (maldevelopment of the pulmonary vasculature), also known as idiopathic pulmonary hypertension. This condition is sometimes referred to as "black lung" PPHN due to the absence of parenchymal abnormalities and diminished pulmonary opacity on chest X-rays. Affected infants typically exhibit abnormal muscularization of the pulmonary arteries, severe hypoxemia, and pulmonary vasoconstriction, with a relatively poor prognosis.
Pulmonary Hypoplasia
Pulmonary hypoplasia resulting from factors such as oligohydramnios or pulmonary arterial obstruction (e.g., polycythemia or hyperviscosity syndrome) can lead to pulmonary hypertension.
Congenital Diaphragmatic Hernia with Pulmonary Hypertension
Congenital diaphragmatic hernia is often accompanied by pulmonary hypoplasia and PPHN. Despite significant improvements in survival rates for other causes of PPHN, the mortality rate associated with diaphragmatic hernia and the requirement for extracorporeal membrane oxygenation (ECMO) remain high.
Alveolar Capillary Dysplasia (ACD)
ACD is a rare condition that involves abnormal distribution and arrangement of pulmonary veins, leading to severe respiratory failure and PPHN. The mortality rate for this condition is extremely high.
Cardiac Dysfunction with Pulmonary Hypertension
Premature closure of the ductus arteriosus in utero can cause hemodynamic changes, leading to postnatal pulmonary hypertension and right heart failure. Additionally, left heart dysfunction can result in pulmonary venous hypertension, which can progress to pulmonary arterial hypertension. The primary treatment in such cases involves improving myocardial function rather than reducing pulmonary vascular resistance.
Perinatal Drug Exposure and PPHN
Prenatal exposure to nonsteroidal anti-inflammatory drugs (NSAIDs), which may lead to in utero closure of the ductus arteriosus, or the use of selective serotonin reuptake inhibitors (SSRIs) during late pregnancy, has been linked to the development of PPHN in newborns.
Pathophysiology
In normal neonates, pulmonary vascular resistance significantly decreases after birth due to the initiation of breathing and a rise in arterial oxygen tension, leading to a drop in pulmonary arterial pressure and an increase in pulmonary blood flow. In PPHN, a range of perinatal factors causes elevated pulmonary arterial pressure, with the key pathophysiological feature being right-to-left shunting of blood through the patent foramen ovale and/or ductus arteriosus. This phenomenon occurs when pulmonary arterial or right heart pressure exceeds systemic pressure. The shunting, characterized by hypoxemic blood bypassing the lungs, results in markedly reduced systemic oxygen saturation. Hypoxemia in PPHN cannot typically be ameliorated by simply increasing oxygen concentration due to the persistence of right-to-left shunting. Furthermore, hypoxemia exacerbates pulmonary vasoconstriction and increases vascular resistance, creating a vicious cycle of pulmonary hypertension, also known as "persistent pulmonary hypertension."
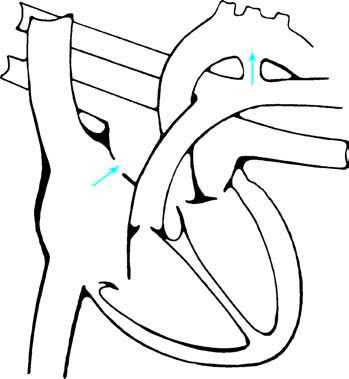
Figure 1 Schematic diagram of right-to-left shunting at the atrial and ductal levels in PPHN
Arrows indicate the direction of shunting.
Multiple pathways regulate changes in pulmonary vascular resistance, including the nitric oxide (NO)-cGMP pathway, prostacyclin-cAMP pathway, endothelial nitric oxide synthase (eNOS) expression, oxidative stress, endothelin-1 (ET-1) levels, and thromboxane levels. Understanding these mechanisms provides potential therapeutic targets for clinical intervention.
Clinical Manifestations
Patients with persistent pulmonary hypertension of the newborn (PPHN) are often full-term, post-term, or near-term infants. A history of perinatal asphyxia, meconium-stained amniotic fluid, or meconium aspiration may be present. Within the first 24 hours after birth, cyanosis may develop following an initial period of respiratory distress. If primary pulmonary disease is present, symptoms and signs of respiratory distress may include tachypnea, intercostal retractions, or grunting. Arterial blood gas analysis often reveals severe hypoxemia, with relatively normal PaCO2 levels. When a neonate presents with severe hypoxemia that is disproportionate to the extent of pulmonary parenchymal disease or the findings on chest X-ray, and conditions like pneumothorax and cyanotic congenital heart disease have been ruled out, PPHN should be considered.
Infants with PPHN typically exhibit pronounced cyanosis, which generally does not improve with oxygen supplementation. On cardiac auscultation, a systolic murmur caused by tricuspid regurgitation may be heard along the left or right lower sternal border. An accentuated second heart sound due to elevated pulmonary arterial pressure may also be apparent. For neonates on mechanical ventilation, if oxygenation becomes unstable without changes to ventilatory settings, the possibility of PPHN should be evaluated. However, it is important to note that oxygen instability can also occur due to ventilation-perfusion mismatch in cases of pulmonary parenchymal disease, making this symptom not specific to PPHN.
Diagnosis
Clinical Presentation
The diagnosis of PPHN should be considered in infants with severe hypoxemia that is inconsistent with the degree of pulmonary disease observed on X-ray. A thorough history and physical examination are essential, but differentiation from cyanotic congenital heart disease is necessary. In cases of PPHN, right-to-left shunting at the atrial or ductal level may be observed. Evidence supporting ductal-level right-to-left shunting includes a postductal (lower limb) arterial oxygen partial pressure (PaO2) that is 10–20 mmHg lower than preductal (right upper limb) PaO2, or a difference in preductal and postductal oxygen saturation of 5%–10% or more (with lower measurements in the lower limbs). Such findings indicate ductal-level shunting. However, if right-to-left shunting occurs only at the atrial level (via the foramen ovale), differences in PaO2 or oxygen saturation may not be observed, and PPHN cannot be ruled out solely based on the absence of such discrepancies.
Echocardiographic Evaluation
Assessing pulmonary arterial pressure is critical in diagnosing PPHN. Two-dimensional echocardiography can exclude cyanotic congenital heart defects. Doppler imaging can detect right-to-left shunting at the ductal and/or atrial levels. The peak velocity of tricuspid regurgitation flow can also be measured, allowing the calculation of pulmonary arterial pressure using the simplified Bernoulli equation: pulmonary arterial pressure = right atrial pressure + 4 × (tricuspid regurgitation velocity)2.
Additional Tests
Brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) levels may be significantly elevated during the acute phase of PPHN, serving as biomarkers for the condition.
Treatment
The severity of PPHN can range from mild hypoxia with mild respiratory distress to severe hypoxemia accompanied by cardiopulmonary instability. The treatment objectives involve reducing pulmonary vascular resistance, maintaining systemic blood pressure, correcting right-to-left shunting, and improving oxygenation. In addition to addressing the underlying disease, general supportive care is essential. This includes providing an optimal thermal environment and nutritional support, minimizing stress and stimuli, and administering sedation and analgesia when necessary. Maintaining internal homeostasis and correcting severe acidosis are important, with the acute-phase blood pH of PPHN maintained above 7.25. Normal blood pressure should be sustained to reduce right-to-left shunting, and in cases of hypotension, volume resuscitation can be implemented using albumin, plasma, blood transfusion, or normal saline. Positive inotropes such as dopamine or dobutamine may also be administered.
Respiratory Support and Optimization of Lung Ventilation
Neonates diagnosed with PPHN generally require mechanical ventilation support to maintain a partial pressure of oxygen (PaO2) between 55–80 mmHg or transcutaneous oxygen saturation (SaO2) at 90%–98%. Both overinflation and atelectasis of the lungs can increase pulmonary vascular resistance, so maintaining optimal lung volumes is crucial. Appropriate levels of positive end-expiratory pressure (PEEP) and mean airway pressure (MAP) should be selected during mechanical ventilation. High-frequency ventilation may also be employed to recruit and reopen more alveoli while minimizing lung injury. For neonates with pulmonary parenchymal diseases such as respiratory distress syndrome (RDS), meconium aspiration syndrome (MAS), or pneumonia, which result in primary or secondary surfactant deficiency and concurrent PPHN, administration of pulmonary surfactant can help recruit and reopen alveoli, thereby improving oxygenation.
Pulmonary Hypertension Reduction with Vasodilators
After implementing adequate alveolar recruitment strategies, including conventional/high-frequency ventilation and surfactant administration, additional vasodilator therapy may be considered based on the infant's oxygenation status, systemic blood pressure, and echocardiographic evaluation of cardiac function.
Inhaled Nitric Oxide (iNO)
A selective pulmonary vasodilator, iNO does not significantly affect systemic blood pressure. It is distributed to ventilated alveoli, which helps improve the ventilation-perfusion ratio. Clinical studies have demonstrated that iNO can enhance oxygenation in PPHN and reduce the need for extracorporeal membrane oxygenation (ECMO). It has become a standard treatment for PPHN in term and near-term neonates. The oxygenation index (OI), defined as FiO2 × mean airway pressure × 100 / PaO2, within the range of 15–25 is considered an indication for iNO use. The gas is typically administered via the mechanical ventilation circuit at an initial concentration of 20 ppm.
Phosphodiesterase-5 (PDE-5) Inhibitors
Under physiological conditions, nitric oxide (NO) induces vasodilation by increasing cyclic guanosine monophosphate (cGMP) levels in vascular smooth muscle cells. cGMP degradation is mediated by PDE-5, and inhibiting PDE-5 decreases cGMP degradation, thereby enhancing its accumulation and promoting pulmonary vasodilation. Sildenafil, a commonly used PDE-5 inhibitor, is administered at a dose of 0.5–2 mg/kg every 6–12 hours via a gastric tube.
Other Vasodilators
Additional agents include endothelin receptor antagonists, which inhibit the vasoconstrictive effects of endothelin, and phosphodiesterase-3 (PDE-3) inhibitors, which increase cyclic adenosine monophosphate (cAMP) levels. Prostacyclins, such as inhaled iloprost and intravenous treprostinil, can also be used for vasodilation in the treatment of PPHN.
Extracorporeal Membrane Oxygenation (ECMO)
For cases of severe hypoxemic respiratory failure and pulmonary hypertension where oxygenation remains refractory to standard respiratory support and vasodilator therapy, ECMO may be utilized. This approach corrects persistent hypoxemia while allowing for reduced ventilatory settings, thereby facilitating pulmonary recovery.