Bronchopulmonary dysplasia (BPD), also referred to as chronic lung disease (CLD), is among the most common and severe respiratory complications in extremely preterm infants. It is characterized by impaired lung development and chronic oxygen dependency, along with distinct clinical, histological, and radiological features. BPD can lead to significant abnormalities in the respiratory and cardiovascular systems as well as developmental impairments, with adverse effects that may persist throughout childhood and even into adulthood.
Etiology, Pathology, and Pathogenesis
BPD arises from a combination of multiple factors, developing on the basis of prematurity and immature lung development. Mechanical ventilation, oxygen toxicity, infections, and other adverse factors collectively contribute to impaired lung growth and injury.
Immature Lung Development
In humans and other mammals, fetal lung development occurs in five stages: embryonic, pseudoglandular, canalicular, saccular, and alveolar stages. In preterm infants born before 28 weeks of gestation, lung development is still at the canalicular stage. Postnatal exposure to adverse environments such as mechanical ventilation, high-concentration oxygen, infection, and inflammatory injury further impairs lung maturation.
Oxygen Toxicity
High-concentration oxygen produces large amounts of toxic reactive oxygen species (ROS) in the body, which disrupt cellular metabolism and result in widespread cellular and tissue damage. These changes include pulmonary edema, fibrin deposition, and reduced activity of pulmonary surfactant (PS). Preterm infants have lower levels and activity of antioxidants, such as enzymes, vitamins C and E, and other free radical scavengers, making them particularly susceptible to oxidative stress.
Ventilator-Induced Lung Injury
This type of injury primarily includes barotrauma, volutrauma, and biotrauma. Immature lung development in preterm infants, characterized by underdeveloped elastic fibers and connective tissue, increases vulnerability. Excessive tidal volume may lead to overdistension of the alveoli, whereas insufficient tidal volume may result in atelectasis. Damage to capillary endothelial cells, alveolar epithelial cells, and the basement membrane allows fluid to leak into the alveolar space, triggering inflammatory responses and the release of pro-inflammatory factors.
Infection and Inflammatory Response
Intrauterine infection is an important contributor to BPD in preterm infants. Maternal infections such as chorioamnionitis, cytomegalovirus (CMV), or Ureaplasma spp. increase the risk of BPD development in neonates, underscoring the roles of intrauterine infection and inflammation in its pathogenesis.
Furthermore, variations in race and genetics affect the incidence and severity of BPD, suggesting that genetic predisposition plays a role. Other contributing factors include symptomatic PDA after birth, excessive fluid intake leading to interstitial pulmonary edema, deficiencies in vitamins A and E, sepsis, and gastroesophageal reflux (GER).
Clinical Manifestations
Population and Risk Factors
BPD most commonly affects preterm infants with gestational ages <28 weeks and birth weights <1,000 g. The smaller the gestational age and birth weight, the higher the incidence rate. A key clinical feature is chronic oxygen dependency, the extent of which is detailed in Table 6-6 and Table 6-7. Preterm infants requiring mechanical ventilation during the neonatal period and failing to wean off the ventilator by the first week of life are at risk for early BPD development. Maternal chorioamnionitis and intrauterine fetal infections are also significant risk factors.
Symptoms and Signs
Symptoms vary depending on disease severity. Common clinical findings include tachypnea, inspiratory retractions, and pulmonary rales, representing symptoms and signs of respiratory insufficiency.
Disease Progression and Complications
Many patients gradually wean off mechanical ventilation or oxygen support over time. However, during the course of the disease, repeated respiratory tract infections, symptomatic PDA, and pulmonary hypertension can exacerbate the condition or lead to death. Severe cases may result in long-term residual chronic respiratory and cardiovascular sequelae. Chronic hypoxemia, increased energy expenditure, and feeding difficulties further contribute to complications such as extrauterine growth restriction (EUGR) and cerebral palsy.
Laboratory and Auxiliary Examinations
Arterial Blood Gas Analysis
Findings typically include hypoxemia, hypercapnia, and respiratory acidosis accompanied by metabolic alkalosis.
Pulmonary Function Testing
Infants with severe BPD at a postmenstrual age (PMA) of 52 weeks often exhibit reduced tidal volumes, increased airway resistance, and decreased dynamic lung compliance. Airway obstruction with uneven ventilation, gas trapping, and hyperinflation may also be observed.
Chest X-rays
Classic radiographic features of BPD include pulmonary hyperinflation, atelectasis, cystic changes, and interstitial pulmonary emphysema. Atypical cases may show only hyperinflation and blurred pulmonary vascular markings. Severe cases with associated pulmonary hypertension may demonstrate an enlarged main pulmonary artery opacity.
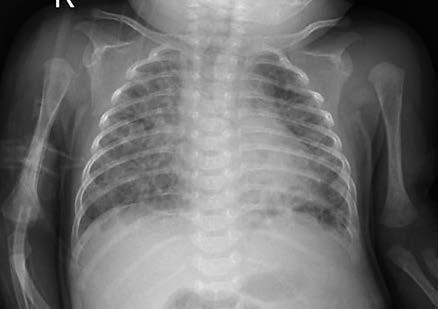
Figure 1 Chest X-ray of a bronchopulmonary dysplasia (BPD) patient
The chest X-ray shows blurred pulmonary markings, areas of pulmonary hyperinflation, and cystic changes.
Pulmonary CT Scans
High-resolution CT scans primarily reveal interstitial lung disease. However, because of radiation exposure concerns, they are no longer used as routine diagnostic tools.
Diagnosis Criteria
Diagnosis criteria for BPD can be found in Table 1.

Table 1 Diagnostic criteria and severity classification of BPD (2001)
A diagnosis of BPD requires oxygen dependency for at least 28 days. The severity classification is evaluated as follows:
- For infants born at <32 weeks of gestation, assessments are conducted at 36 weeks postmenstrual age (PMA) or at hospital discharge, whichever occurs first.
- For infants born at ≥32 weeks of gestation, assessments are conducted at postnatal day 56 or at hospital discharge, whichever occurs first.
BPD severity classification standards are listed in Table 2.
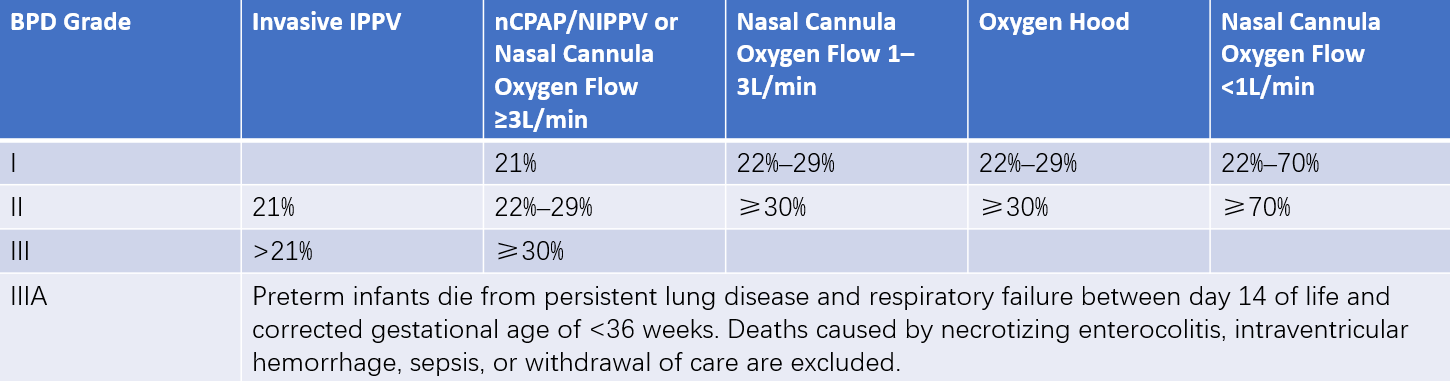
Table 2 Severity grading criteria of BPD (2018)
Note: Percentages in the table refer to the concentration of inhaled oxygen.
Treatment
Nutritional Support
Chronic hypoxia and increased respiratory workload require adequate energy and protein intake for children with BPD. Severe cases may need caloric intake of 120–150 kcal/(kg·d). Vitamin A supplementation facilitates alveolar epithelial cell proliferation. Anemia should be actively corrected, either through blood transfusion or by using erythropoiesis-stimulating agents to reduce the frequency of transfusions.
Fluid Restriction
Children with BPD often experience fluid balance abnormalities in the lungs. Even normal fluid intake may lead to interstitial and alveolar edema. Fluid intake and sodium levels should be carefully controlled. However, excessive fluid restriction may result in malnutrition, potentially delaying the process of alveolarization. Diuretics may be used when necessary.
Respiratory Support
For severe BPD, mechanical ventilation parameters, including tidal volume, inspiratory time, and PEEP (positive end-expiratory pressure), are adjusted to align with the pathophysiological characteristics of the condition. Higher parameter settings may sometimes be required. After hospital discharge, home oxygen therapy may be continued based on oxygen saturation (SpO2) monitoring, with a target SpO2 of 92%–94%.
Anti-Inflammatory Therapy
Glucocorticoids possess anti-inflammatory effects that reduce bronchospasm, pulmonary edema, and lung fibrosis, promote antioxidant activity, and improve respiratory function. Their use can facilitate weaning from mechanical ventilation and reduce the incidence of BPD. However, clinical studies have found that glucocorticoids may increase the risk of mortality, suppress head growth, and potentially heighten the risk of cerebral palsy. When selecting glucocorticoid therapy, careful assessment is required to balance the benefits of reducing oxygen dependency against the risks associated with adverse effects, particularly on the nervous system.
Infection Control
Secondary bacterial, viral, or fungal infections often exacerbate the disease course. Pathogens should be identified, and targeted antibiotic treatment should be initiated as needed. Respiratory syncytial virus (RSV) is a leading cause of recurrent respiratory tract infections after discharge in children with BPD. The American Academy of Pediatrics recommends passive immunization as a preventive measure during the RSV season for children with severe BPD.
Other Therapies
Caffeine citrate is used to prevent and treat apnea of prematurity. In preterm infants with birth weights below 1,250 g, caffeine has been shown to prevent or reduce the development of BPD. Other treatments, including inhaled bronchodilators, exogenous surfactant, antioxidants, inhaled nitric oxide (NO), and stem cell therapy, are currently under investigation.
Since there are no highly effective treatment options available, preventing the occurrence of BPD remains more important than treating it. Measures for prevention include avoiding preterm birth, administering antenatal corticosteroids to mothers at risk of preterm delivery, early postnatal stabilization within the first hour, protective ventilation strategies, and reducing infection risks.
Prognosis
As survival rates for extremely preterm infants have increased, the incidence of BPD has also risen, and the associated mortality rate and long-term outcomes remain concerning. The mortality rate for severe BPD is approximately 25%. Among survivors, the rehospitalization rate during the first year reaches 50%, with repeated lower respiratory tract infections as the primary cause. Additionally, children with BPD are 2–3 times more likely than their peers to experience delayed neurodevelopment.