Respiratory distress syndrome (RDS) in neonates is a clinical syndrome characterized by the onset of respiratory distress shortly after birth, with progressive worsening, primarily caused by a deficiency of pulmonary surfactant (PS). Due to the formation of hyaline membranes in the lungs as a pathological hallmark, the condition is also referred to as hyaline membrane disease (HMD). RDS is more common in preterm infants, with higher incidence rates in those of younger gestational age. Advances such as antenatal corticosteroid administration and the early application of PS and continuous positive airway pressure (CPAP) have not only reduced the incidence of RDS in preterm infants but also altered the typical manifestations and severity of the condition.
Composition and Function of Pulmonary Surfactant (PS)
Pulmonary surfactant is a phospholipid-protein complex synthesized and secreted by type II alveolar epithelial cells. Phospholipids account for approximately 80% of its composition, proteins for about 13%, and small amounts of neutral lipids and carbohydrates comprise the remainder. Among the phospholipids, phosphatidylcholine (PC), also known as lecithin, is the key substance responsible for surfactant activity. Its production begins at 18–20 weeks of gestation, increases gradually, and surges rapidly between 35–36 weeks, reaching levels indicative of lung maturity. Another important phospholipid, phosphatidylglycerol (PG), remains at low concentrations before 26–30 weeks but rises in parallel with PC, peaking at 36 weeks before slightly declining to approximately half of its peak concentration at term.
Other phospholipids include sphingomyelin, whose concentration remains relatively constant with a slight peak around 28–30 weeks of gestation. Thus, the lecithin/sphingomyelin (L/S) ratio in amniotic fluid or tracheal aspirates is an important marker for evaluating fetal or neonatal lung maturity.
PS also contains surfactant proteins (SP), including SP-A, SP-B, SP-C, and SP-D, which bind to phospholipids and enhance their surfactant activity. Neutral lipids primarily consist of cholesterol, triglycerides, and free fatty acids, whose functions remain unclear. Carbohydrates such as mannose and trehalose are also present, often in combination with SP proteins.
Pulmonary surfactant forms a film on the surface of alveoli, where its primary function is to reduce surface tension, thereby preventing alveolar collapse at the end of expiration and maintaining functional residual capacity (FRC). Additional benefits include stabilizing alveolar pressure, improving lung compliance, and reducing fluid leakage from capillaries into the alveoli. Furthermore, SP-A and SP-D may play roles in immune regulation within the respiratory tract.
Etiology
The fundamental cause of RDS is a deficiency in pulmonary surfactant production.
Preterm Birth
The lower the gestational age, the lower the amount of synthesized and secreted PS, and the higher the incidence of RDS. Among preterm infants born at fewer than 30 weeks of gestation, the incidence of RDS exceeds 70%, whereas it decreases to 1%–5% in preterm infants born after 36 weeks of gestation.
Infants of Diabetic Mothers (IDM)
Infants born to mothers with diabetes are also at increased risk of RDS, with rates 5–6 times higher compared to non-diabetic pregnancies. This is due to the antagonistic effect of high circulating insulin levels on the stimulation of PS synthesis by adrenal corticosteroids.
Elective Cesarean Delivery
RDS incidence has shown an increasing trend among infants born via elective cesarean section. This is mainly associated with deliveries occurring prior to the onset of labor, resulting in the absence of stress hormones such as catecholamines and adrenal corticosteroids, which are known to enhance PS synthesis and secretion.
Other Factors
Perinatal asphyxia, hypothermia, placenta previa, placental abruption, maternal hypotension, and conditions leading to reduced fetal blood volume have all been implicated as triggers for RDS. Some studies have also identified genetic mutations or deficiencies in SP-A or SP-B genes, which impair surfactant function, making affected infants more susceptible to RDS regardless of whether they are preterm or full-term.
Pathogenesis
The reduced concentration of pulmonary surfactant leads to increased alveolar surface tension, decreased FRC at the end of expiration, and a tendency for alveolar collapse. Pulmonary dysfunction in RDS primarily manifests as decreased lung compliance, impaired ventilation-perfusion (V/Q) ratio, gas diffusion abnormalities, and increased work of breathing. These abnormalities result in hypoxemia, metabolic acidosis, and respiratory acidosis due to ventilatory impairment.
Hypoxemia and acidosis further increase the permeability of pulmonary capillaries, resulting in fluid leakage into the alveolar and interstitial spaces. This leads to pulmonary interstitial edema and the deposition of fibrin on the alveolar surfaces, forming eosinophilic hyaline membranes. The formation of hyaline membranes exacerbates gas exchange impairment, worsens hypoxemia and acidosis, and further suppresses PS synthesis, creating a vicious cycle.
Additionally, severe hypoxemia and mixed acidosis increase the risk of developing persistent pulmonary hypertension of the newborn (PPHN).
Clinical Manifestations
Respiratory distress syndrome (RDS) is most frequently observed in preterm infants, with symptoms of respiratory distress appearing shortly after birth (typically within 6 hours) and progressively worsening. The primary clinical features include tachypnea (respiratory rate > 60/min), expiratory grunting, cyanosis, nasal flaring, and chest indrawing. In severe cases, symptoms may escalate to shallow breathing, irregular respiratory rhythms, apnea, and limb hypotonia. Expiratory grunting is a hallmark of this condition, caused by partial closure of the glottis during exhalation, which creates positive pressure and prevents alveolar collapse. Physical examination often reveals a flattened chest with reduced breath sounds in both lungs owing to decreased tidal volumes; fine crackles may be auscultated if there is alveolar exudation.
As the condition improves, pulmonary compliance increases and pulmonary vascular resistance decreases. During the recovery phase, approximately 30%–50% of infants may develop patent ductus arteriosus (PDA). If the shunt volume through the PDA is substantial, complications such as heart failure and pulmonary edema may arise. Therefore, in the recovery phase of RDS, if an infant's primary condition has significantly improved yet suddenly exhibits increased oxygen demand, unexplained or refractory metabolic acidosis, feeding difficulties, apnea, general pallor with mottling, and a rapidly enlarging liver, PDA should be considered. Additional findings that support PDA include widened pulse pressure, bounding pulses, tachycardia or bradycardia, increased precordial activity, and the presence of a systolic or continuous murmur at the left second intercostal space along the sternal border.
The severity of RDS typically peaks 24–48 hours after birth, with a high mortality rate during this period. However, infants who survive beyond three days generally experience disease resolution due to increased lung maturity. It is worth noting that widespread use of pulmonary surfactant in recent years has led to milder disease severity and shorter disease courses in RDS. Among preterm infants who have not received surfactant therapy, the onset of respiratory distress after 12 hours of life is less likely to be due to RDS.
With the rising prevalence of elective cesarean sections, the incidence of RDS in term infants has been increasing. Compared to preterm infants, the onset of symptoms in term infants is slightly delayed, with more severe clinical manifestations and a higher likelihood of complications such as persistent pulmonary hypertension of the newborn (PPHN). Additionally, the effectiveness of surfactant therapy is less pronounced in term infants compared to preterm infants.
Auxiliary Examinations
Laboratory Tests
Blood Gas Analysis
This is the most commonly utilized diagnostic method, in which findings typically demonstrate decreased pH and arterial oxygen partial pressure (PaO2), increased arterial carbon dioxide partial pressure (PaCO2), and reduced bicarbonate levels.
Other Methods
Historically, tests such as foam test and measurements of lecithin-to-sphingomyelin (L/S) ratios in amniotic fluid or tracheal aspirates were used to assess lung maturity. However, these methods are now rarely applied in clinical practice.
X-Ray Examination
Radiographic imaging provides characteristic findings and remains the gold standard for diagnosing RDS:
- A uniform decrease in lung transparency with a diffuse, evenly distributed fine reticulogranular pattern described as a ground-glass appearance.
- On the background of diffuse alveolar collapse (white), the presence of air-filled branching bronchi (black) is visible, representing the air bronchogram sign.
- In cases of severe RDS, the lung fields may appear completely white, with the heart and diaphragm borders obscured, referred to as white lung.
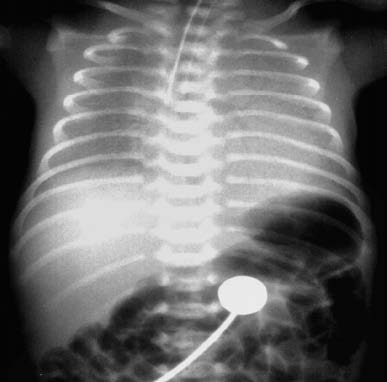
Figure 1 Chest X-ray of an infant with RDS
There is a marked reduction in lung field transparency with ground-glass changes. Air bronchograms are visible near both hila, and the borders of the heart on both sides appear blurred.
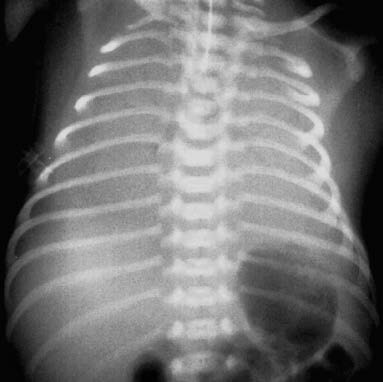
Figure 2 Chest X-ray of an infant with RDS
There is uniform and consistent reduction in lung field transparency without normal pulmonary vascular markings. Air bronchograms are visible within the lung fields, while the borders of the heart, diaphragm, and costophrenic angles are indistinct.
Ultrasound Examination
Color Doppler ultrasonography may aid in definitively diagnosing patent ductus arteriosus (PDA). Additionally, ultrasonography can help differentiate RDS from transient tachypnea of the newborn (wet lung).
Differential Diagnosis
Transient Tachypnea of the Newborn (TTN)
Also known as wet lung, TTN is common in term infants or those delivered by cesarean section. It results from delayed absorption and clearance of lung fluid and is a self-limiting condition. Symptoms, including tachypnea (respiratory rate > 60–80/min), appear within a few hours of birth. General condition and responsiveness are usually better compared to RDS, but severe cases may also exhibit cyanosis and grunting. On auscultation, decreases in breath sounds and fine crackles are common. X-ray findings may include hyperaeration, thickened perihilar markings, spot-like infiltrates, and interlobar fluid lines. Symptoms generally resolve within 2–3 days, and treatment focuses mainly on supportive care.
Group B Streptococcal (GBS) Pneumonia
This is an in utero infectious pneumonia caused by group B streptococcal sepsis. Clinical presentations and X-ray findings may closely resemble those of RDS. However, maternal history often reveals late pregnancy infection, premature rupture of membranes, or foul-smelling amniotic fluid. Positive GBS cultures from maternal rectal or cervical swabs further support the diagnosis. Laboratory investigations in the infant, including peripheral blood counts, elevated C-reactive protein (CRP), and positive blood cultures, provide additional evidence of infection. Disease progression differs from RDS, and antibiotic therapy is effective for GBS pneumonia.
Diaphragmatic Hernia
Infants often present shortly after birth with paroxysmal respiratory distress and cyanosis. Clinical findings include a concave abdomen, diminished or absent breath sounds over the affected side of the chest, and bowel sounds auscultated in the chest. X-rays typically reveal air-filled bowel loops or gastric bubbles within the affected hemithorax, atelectasis of the lung on the affected side, and mediastinal shift to the contralateral side. In some cases, prenatal diagnosis is possible through fetal ultrasonography.
Treatment
The treatment goal is to ensure adequate ventilation-exchange function until endogenous pulmonary surfactant (PS) production increases and the symptoms of RDS resolve. Mechanical ventilation and the administration of PS are crucial therapeutic approaches.
General Management
Thermal Regulation
The infant is placed in an incubator or under a radiant heater to maintain a skin temperature of approximately 36.5°C.
Monitoring
Continuous monitoring includes body temperature, respiratory rate, heart rate, blood pressure, and arterial blood gases.
Fluid and Nutritional Support
Fluid intake on the first day is generally 70–80 ml/(kg·d) and gradually increases thereafter. Excessive fluid intake should be avoided to reduce the risk of patent ductus arteriosus (PDA) or pulmonary edema.
Antibiotics
Antibiotics are recommended as a routine measure until bacterial infection or sepsis is excluded in RDS patients.
Oxygen Therapy and Assisted Ventilation
Oxygen Administration
For mild cases, oxygen may be administered through nasal cannula, face mask, head box, or nasal prongs. The target is to maintain PaO2 at 50–80 mmHg (6.7–10.6 kPa) and transcutaneous oxygen saturation (TcSO2) between 90% and 95%.
Continuous Positive Airway Pressure (CPAP)
Early CPAP application is beneficial for preterm neonates with high-risk factors for RDS as it reduces the need for PS administration and endotracheal intubation. For confirmed cases of RDS, the combination of CPAP and PS is considered the most effective treatment strategy.
Method
Nasal prongs are the most commonly used method, although nasal masks, face masks, and nasopharyngeal tubes are also options.
Parameters
Pressure settings generally range from 4 to 8 cmH2O. The gas flow rate should be at least three times the infant's minute ventilation or a minimum of 5 L/min. FiO2 is adjusted based on SaO2 levels.
In addition to CPAP, other non-invasive ventilation modalities, such as nasal intermittent positive pressure ventilation (NIPPV), bi-level positive airway pressure (BiPAP), heated humidified high-flow nasal cannula (HFNC), and non-invasive high-frequency ventilation (NIHFV), are also used in the clinical management of RDS. However, the long-term benefits and advantages of these methods compared to conventional CPAP require further research and validation.
Conventional Mechanical Ventilation (CMV)
The routine use of PS in RDS has led to lower ventilator settings and shorter durations of mechanical ventilation.
Indications
There is no universally accepted set of criteria for initiating mechanical ventilation. Proposed indications include the following:
- FiO2 ≥ 0.6 with PaO2 < 50 mmHg (6.7 kPa) or TcSO2 < 85% (excluding cyanotic congenital heart disease).
- PaCO2 > 60–70 mmHg (7.8–9.3 kPa) with pH < 7.25.
- Severe, recurrent, or treatment-resistant apnea.
Meeting any of the above criteria warrants mechanical ventilation via endotracheal intubation.
Parameters
Peak inspiratory pressure (PIP) should be set to achieve visible chest rise, generally at 20–25 cmH2O. Positive end-expiratory pressure (PEEP) is usually maintained at 4–6 cmH2O. Respiratory rates (RR) range from 20 to 40 breaths/min, with an inspiratory time (Ti) between 0.3 and 0.4 seconds. FiO2 is adjusted to achieve target TcSO2 levels, with arterial blood gas analysis performed 15–30 minutes later to guide further adjustments.
High-Frequency Ventilation (HFV)
For RDS cases where CMV has failed, HFV may serve as a rescue therapy. Studies suggest that early application of HFV as a first-line treatment for RDS may reduce the incidence of bronchopulmonary dysplasia (BPD), shorten hospital stays, decrease PS usage, and facilitate earlier extubation.
Surfactant Replacement Therapy
Surfactant replacement therapy significantly reduces RDS mortality rates and the risk of pneumothorax. It also improves lung compliance and ventilation-exchange function, enabling lower ventilator settings. Natural surfactants derived from porcine or bovine lungs remain the most commonly used products in clinical practice, although synthetic and recombinant forms are also available.
Indications
Surfactant therapy is indicated when nCPAP pressure is ≥ 6 cmH2O and FiO2 > 0.30. Rapidly progressing or moderate-to-severe RDS should prompt immediate endotracheal intubation for surfactant administration.
Timing
For extremely preterm infants who have not received antenatal corticosteroids and require stabilization via intubation, surfactant administration should occur promptly in the delivery room. For confirmed cases of RDS, earlier administration yields better outcomes. Additional doses (a second or third dose) may be necessary in cases of ongoing RDS progression, such as persistent oxygen dependence or the need for mechanical ventilation.
Dosage
Each surfactant product has a recommended dose. Initial doses of 100–200 mg/kg are commonly reported, with subsequent doses of 100 mg/kg if needed. Initial doses of 200 mg/kg appear to be more effective than 100 mg/kg for confirmed RDS.
Administration Method
After preparation, the surfactant (dilution required for dry powder formulations) is gently shaken and slowly instilled into the lungs via an endotracheal tube. Minimally invasive techniques, such as less invasive surfactant administration (LISA) and minimally invasive surfactant treatment (MIST), have been developed. These methods involve the use of a thin catheter inserted into the trachea under continuous nCPAP without conventional intubation, allowing slow surfactant administration.
Closure of Patent Ductus Arteriosus (PDA)
Conservative Management
Ensuring adequate pulmonary oxygenation is crucial.
Fluid intake is typically limited to 80–100 ml/(kg·d). If phototherapy is required, fluid volumes may be increased to 100–120 ml/(kg·d).
Packed red blood cell transfusion is administered to maintain a hematocrit > 35%.
During mechanical ventilation, maintaining an appropriate level of positive end-expiratory pressure (PEEP) can reduce left-to-right shunting and enhance systemic circulation.
Diuretics are administered in cases with evidence of fluid retention.
Pharmacological Closure
Commonly used medications include the following:
Indomethacin
This is a non-selective cyclooxygenase (COX) inhibitor that inhibits both COX-1 and COX-2, inducing PDA closure in 66%–98.5% of cases. Intravenous formulations are preferred due to fewer gastrointestinal side effects compared to oral formulations. The usual dosage is 0.2 mg/kg every 12–24 hours for three doses. Significant contraction effects are often observed within two hours of the first dose. Common adverse effects include gastrointestinal bleeding or perforation, renal dysfunction, hyponatremia, and transient reductions in organ blood flow.
Ibuprofen
This is another non-selective cyclooxygenase inhibitor that promotes PDA closure by inhibiting the prostaglandin pathway derived from cyclooxygenase-2 (COX-2)-mediated arachidonic acid metabolism. Extensive clinical evidence demonstrates that ibuprofen is equally as effective as indomethacin for closing PDA. The recommended dosage is an initial dose of 10 mg/kg, followed by two additional doses of 5 mg/kg each, given at 24-hour intervals. Intravenous formulations are preferred, but the effectiveness of oral formulations is also widely recognized. Because ibuprofen has a more pronounced effect on COX-2 and a weaker effect on COX-1, its impact on organ blood flow, especially renal side effects, is less significant.
Acetaminophen
Acetaminophen has also been used for PDA closure, but its efficacy and safety require further validation.
Surgical Treatment
Surgical ligation remains the most reliable method for PDA closure. Surgical intervention is recommended when a second course of pharmacological treatment fails, or if PDA persists with significant left-to-right shunting. It is particularly indicated for infants dependent on respiratory support, those with worsening pulmonary conditions, or those with contraindications to pharmacological therapy (e.g., extremely low birth weight infants). Potential risks of surgical ligation include complications such as pneumothorax, chylothorax, scoliosis, and left vocal cord paralysis.
Prevention
Pregnant women at risk of preterm birth before 30 weeks of gestation should be transferred to perinatal centers equipped to manage RDS.
Antenatal corticosteroid therapy is recommended for all pregnant women at risk of preterm delivery before 34 weeks of gestation.
Elective cesarean sections before 39 weeks of gestation are not recommended unless medically indicated.