Meconium aspiration syndrome (MAS), also referred to as meconium aspiration pneumonia, occurs when a fetus inhales amniotic fluid mixed with meconium either in utero or during delivery. The pathological features include mechanical airway obstruction and chemical inflammation of lung tissue. Affected infants exhibit respiratory distress immediately after birth and are at risk of complications such as pulmonary hypertension and air leaks. MAS primarily occurs in term or post-term infants. The incidence of meconium-stained amniotic fluid during delivery is around 8%–25%, with approximately 5% developing MAS.
Etiology and Pathophysiology
Meconium Aspiration
Fetal hypoxia during pregnancy or delivery decreases blood flow to the intestines and skin, activates the vagus nerve, and leads to intestinal wall ischemia and increased peristalsis. This process causes relaxation of the anal sphincter and the release of meconium into the amniotic fluid. Hypoxia may also stimulate fetal respiratory movements, resulting in aspiration of meconium into the trachea or lungs. Some infants inhale meconium immediately after delivery when effective respiration begins. The incidence of MAS is correlated with gestational age, with rates exceeding 30% in infants with a gestational age over 42 weeks, less than 2% in those under 37 weeks, and exceedingly rare in those under 34 weeks, where meconium-stained amniotic fluid is uncommon.
Uneven Airway Obstruction
The primary pathological change in MAS is mechanical obstruction of the airways caused by meconium, resulting in the coexistence of atelectasis, emphysema, and normal alveoli. The relative proportion of these states determines the severity of the clinical presentation in affected infants.
Atelectasis
Complete obstruction of small airways by large meconium particles leads to absorption of air in downstream alveoli, resulting in atelectasis. This causes ventilation/perfusion mismatch, increases intrapulmonary shunting, and leads to hypoxemia.
Pulmonary Emphysema
Partial obstruction of small airways by viscous meconium creates a "check-valve" effect, in which air enters the alveoli during inspiration when the small airway dilates but cannot fully exit during expiration due to airway blockage. This results in emphysema, reduced alveolar ventilation, and CO2 retention. If emphysematous alveoli rupture, air leak complications such as interstitial emphysema, pneumomediastinum, or pneumothorax may occur.
Normal Alveoli
Some alveoli remain free of meconium, and their ventilation and gas exchange function may compensate for impaired areas.
Chemical Inflammation of Lung Tissue
Within 12–24 hours of meconium aspiration, local lung tissue may develop chemical inflammation and interstitial emphysema due to irritation from bile salts and other constituents in meconium. Additionally, meconium can promote bacterial proliferation, increasing the risk of secondary bacterial pneumonia.
Pulmonary Hypertension
Pulmonary hypertension is frequently observed in term infants, with about one-third of MAS cases exhibiting varying degrees of pulmonary hypertension. The underlying causes include atelectasis, emphysema, and lung inflammation induced by meconium, as well as secondary inactivation of pulmonary surfactant. Hypoxemia and mixed acidosis further exacerbate the condition, preventing pulmonary vascular resistance from adapting to postnatal changes and leading to persistent pulmonary hypertension of the newborn (PPHN).
Other Mechanisms
Meconium can inactivate surfactant proteins in the lungs, reducing the production of SP-A and SP-B and impairing lung compliance. This exacerbates alveolar atelectasis and worsens ventilation and gas exchange dysfunction. The degree of surfactant inhibition correlates with the volume of aspirated meconium.
Clinical Manifestations
MAS is more common in term and post-term infants, often associated with a history of fetal distress and/or birth asphyxia. Symptoms vary in severity depending on the amount and consistency of aspirated amniotic fluid (e.g., suspended meconium or thick meconium clumps). Small amounts of evenly mixed meconium may produce no or only mild symptoms, whereas large volumes of thick meconium can result in stillbirth or death shortly after birth.
Meconium-Stained Amniotic Fluid
This is a critical diagnostic criterion:
- Amniotic fluid is visibly stained with meconium during delivery.
- Meconium staining is observed on the infant's skin, umbilical cord, or fingernail/toenail beds.
- Meconium-containing material is identified in suctioned secretions from the mouth or nasal cavity.
- Meconium is detected in aspirated contents from the glottis or trachea during intubation, confirming the diagnosis.
Respiratory Symptoms
Respiratory distress begins immediately after birth and worsens 12–24 hours later as meconium enters the distal airways. Symptoms include tachypnea (usually >60 breaths/min), cyanosis, nasal flaring, and intercostal or subcostal retractions. Some infants also exhibit expiratory grunting. Physical examination may show a barrel-shaped chest with diminished breath sounds. Early auscultatory findings include coarse crackles or rhonchi, which later transition to fine crackles. Sudden worsening of respiratory distress and significantly diminished breath sounds suggest air leakage, such as tension pneumothorax, which can occur in severe cases.
PPHN
Persistent and severe cyanosis is a hallmark clinical feature of MAS complicated by PPHN. Cyanosis worsens during episodes of crying, feeding, or agitation. The severity of cyanosis often does not correspond to the extent of lung symptoms (i.e., cyanosis is severe despite mild pulmonary findings). Some infants may present with a systolic murmur at the second intercostal space along the left sternal border. Shock and heart failure may develop in severe cases.
Severe MAS may also be associated with complications such as polycythemia, hypoglycemia, hypocalcemia, hypoxic-ischemic encephalopathy (HIE), multi-organ dysfunction, and pulmonary hemorrhage.
Auxiliary Examinations
Laboratory Tests
Arterial blood gas analysis often reveals decreased pH, reduced PaO2, and elevated PaCO2. Additional evaluations include complete blood count, blood glucose, blood calcium levels, and relevant biochemical tests. Bacteriological cultures of aspirates from the trachea and blood can also be conducted.
X-Ray Examination
Findings include hyperinflation of the lungs with segmental or lobular atelectasis. Diffuse infiltrates may also be observed, with potential complications such as pneumomediastinum or pneumothorax. These changes usually become more prominent within 12–24 hours after birth. In some cases of MAS, chest radiographs may not correlate well with clinical severity. Other findings might include increased and blurred lung markings, fine patchy shadows, increased lung field transparency, and thickening of the right horizontal fissure.
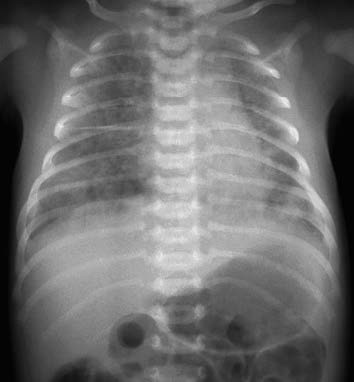
Figure 1 X-ray of MAS
Ultrasound Examination
Color Doppler ultrasonography can assess and monitor pulmonary artery pressure. Identifying right-to-left shunting at the level of the ductus arteriosus or foramen ovale, as well as tricuspid regurgitation, can further support the diagnosis of persistent pulmonary hypertension of the newborn (PPHN).
Diagnosis
The diagnosis is confirmed by a clear history of meconium-stained amniotic fluid aspiration (as evidenced by the presence of meconium in aspirated material from the glottis or trachea during intubation), the onset of respiratory distress shortly after birth, and characteristic changes seen on chest X-rays.
Treatment
Facilitation of Meconium Removal from the Trachea
In severe cases of MAS occurring shortly after birth, suctioning via endotracheal intubation may help alleviate airway obstruction caused by meconium. Experimental studies in animals suggest that even up to 4 hours after aspiration, some of the meconium can still be removed from the airways.
Symptomatic Treatment
Oxygen Therapy
Oxygen therapy becomes necessary when PaO2 is <50 mmHg (6.7 kPa) or TcSO2 is <90%. The method of oxygen delivery depends on the degree of hypoxia and may include nasal cannulas, headboxes, or face masks. The goal is to maintain PaO2 at 50–80 mmHg (6.7–10.6 kPa) or TcSO2 at 90%–95%. The use of warmed and humidified oxygen is preferred when available, as it may help in the clearance of meconium.
Mechanical Ventilation
Continuous Positive Airway Pressure (CPAP)
CPAP can be tried when FiO2 requirements exceed 0.4. The pressure settings need to be individualized, usually 4–5 cmH2O. However, caution is advised if chest examination or radiographic findings indicate overinflation, as CPAP may exacerbate gas trapping and increase the risk of air leak complications.
Conventional Mechanical Ventilation (CMV)
CMV is indicated if FiO2 exceeds 0.6, TcSO2 falls below 85%, or PaCO2 exceeds 60 mmHg with a pH <7.25. To minimize gas trapping and air leaks, moderate respiratory rates (40–60 breaths/min), the lowest effective peak inspiratory pressure (PIP) to ensure chest movement, and low-to-moderate levels of positive end-expiratory pressure (PEEP) (3–5 cmH2O) are generally preferred, along with adequate exhalation times.
High-Frequency Ventilation (HFV)
HFV utilizes rapid airflow delivery with small tidal volumes to maintain sustained lung inflation and increase lung volume. Among HFV methods, high-frequency oscillatory ventilation (HFOV) is most commonly used for neonates and is widely applied in MAS management. HFV is the treatment of choice when MAS is complicated by severe air leaks or PPHN, especially when inhaled nitric oxide (NO) is required in combination.
Extracorporeal Membrane Oxygenation (ECMO)
ECMO may be considered for critically ill MAS patients when HFV fails and for severe PPHN cases unresponsive to conventional treatments.
Pulmonary Surfactant Therapy
Secondary inactivation of pulmonary surfactant occurs in MAS. Studies have confirmed that exogenous surfactant supplementation effectively improves lung compliance and oxygenation. This therapy is indicated in severe MAS cases and shows enhanced efficacy when combined with HFV or inhaled NO. However, further randomized controlled trials are needed for conclusive evidence.
Other Treatments
Fluid Restriction
Severe cases, often accompanied by pulmonary edema or heart failure, warrant appropriate fluid intake restrictions.
Antibiotics
The necessity of prophylactic antibiotics remains controversial. Nevertheless, in cases with secondary bacterial infection, broad-spectrum antibiotics are common initial agents, adjusted based on blood culture, tracheal aspirate results, and sensitivity testing.
Circulatory Support
Symptoms such as hypothermia, pallor, or hypotension indicating shock may require volume expansion with normal saline or plasma. Vasoactive agents, such as dopamine or dobutamine, may be selectively administered.
Sedatives and Muscle Relaxants
These may be used in larger neonates to reduce ventilator dyssynchrony and excessive ventilation caused by "check-valve" effects, thereby lowering the risk of air leaks.
Other Supportive Measures
Thermal regulation, sedation, adequate caloric intake, and maintenance of normal blood glucose and electrolyte levels are essential components of care.
Prevention
Efforts to prevent fetal distress and birth asphyxia are crucial. Clearing meconium from the nasal and oral cavities when amniotic fluid is meconium-stained—before the delivery of the shoulders and chest—is no longer recommended. Evaluation of neonatal vitality is emphasized. If the newborn is vigorous (defined by regular breathing, good muscle tone, and a heart rate >100 beats/min), observation without immediate tracheal suctioning is advised. In non-vigorous newborns, tracheal intubation can be performed to suction meconium. Positive pressure ventilation should generally be avoided before clearing meconium from the airways.