Neonatal hypoxic-ischemic encephalopathy (HIE) refers to brain injury in neonates with a gestational age of ≥35 weeks caused by partial or complete hypoxia and reduced or interrupted cerebral blood flow during the perinatal period. It is characterized by specific neuropathological and pathophysiological changes along with clinical signs of encephalopathy. Among affected neonates, 15%–20% die during the neonatal period, and 20%–30% of survivors may have varying degrees of neurological sequelae.
Etiology
Any factor impairing maternal-fetal blood circulation or gas exchange, leading to reduced blood oxygen levels, can result in asphyxia. Common causes include:
- Maternal factors: Preeclampsia, massive hemorrhage, cardiopulmonary diseases, severe anemia, or shock.
- Placental factors: Abruptio placentae, placenta previa, placental insufficiency, or structural abnormalities.
- Fetal factors: Intrauterine growth restriction (IUGR), prematurity, post-term pregnancy, or congenital malformations.
- Umbilical cord factors: Prolapse, compression, knots, or nuchal cord (cord around the neck).
- Labor-related factors: Prolonged labor, precipitous labor, abnormal fetal presentations, surgical interventions, or anesthetic use.
- Neonatal factors: Severe hypoxia caused by respiratory and circulatory impairments such as repeated apnea, respiratory distress syndrome, bradycardia, heart failure, shock, or polycythemia.
Pathogenesis
Alterations in Cerebral Blood Flow
During partial or chronic hypoxia-ischemia, blood flow is redistributed within the body to ensure adequate perfusion of vital organs like the heart and brain, at the expense of less critical organs such as the lungs, kidneys, and gastrointestinal tract. As hypoxia persists, this compensatory mechanism is lost; cardiac dysfunction and systemic hypotension lead to a sharp decline in cerebral blood flow. A second redistribution of blood flow occurs, reducing perfusion to cerebral hemispheres while prioritizing regions with higher metabolic demands, such as the basal ganglia, brainstem, thalamus, and cerebellum. This process causes damage to the parasagittal cortex and underlying white matter (watershed areas between the anterior, middle, and posterior cerebral arteries). In cases of acute and complete asphyxia, such compensatory mechanisms do not occur, and injury is localized primarily to metabolically active regions like the basal ganglia while sparing the cerebral cortex and, often, other organs. This phenomenon, where some regions of the brain are inherently more vulnerable due to their unique characteristics, is known as "selective vulnerability."
Impaired Cerebral Autoregulation
Cerebral autoregulation maintains relatively stable cerebral blood flow over a range of blood pressures. Hypoxia-ischemia disrupts this mechanism, resulting in "pressure-passive" cerebral blood flow. High blood pressure causes hyperperfusion, exacerbating ischemia-reperfusion injury and potentially leading to intracranial vascular rupture and hemorrhage. Low blood pressure reduces cerebral blood flow, further worsening ischemic injury.
Metabolic Changes in Brain Tissue
During hypoxia-ischemia, anaerobic glycolysis increases, leading to lactate accumulation and a sharp decline in energy production within brain tissue. This process triggers an energy failure cascade, resulting in neuronal death:
- Insufficient function of sodium-potassium pumps and calcium pumps on cell membranes allows Na+ and water to enter cells, causing cytotoxic cerebral edema.
- Abnormal activation of calcium channels leads to excessive Ca²⁺ influx into cells, causing irreversible neuronal damage.
- Ischemia-reperfusion generates reactive oxygen species (ROS) in large quantities.
- During prolonged energy failure, excitatory amino acids—especially glutamate—accumulate extracellularly, exerting neurotoxic effects and further amplifying the biochemical damage.
Primary and Secondary Energy Failure
Hypoxic-ischemic events constitute the primary phase of cellular damage as reduced blood flow and oxygen delivery activate harmful biochemical cascades, causing cytotoxic edema and cell death. After resuscitation, cerebral perfusion and oxygenation are restored. However, a secondary energy failure occurs 6–12 hours post-insult, primarily driven by mitochondrial dysfunction, which plays a critical role in neuronal apoptosis. Cytochrome c is released from mitochondria into the cytoplasm, initiating caspase activation and eventually triggering programmed cell death. The "latent phase" between the two energy failures represents the therapeutic "window of opportunity," during which neuroprotective interventions can be most effectively employed to mitigate brain injury.
Pathological Changes
The extent, distribution, and type of brain damage largely depend on the maturity of brain tissue, severity of injury, and duration of hypoxic-ischemic events.
- Cerebral edema: Represents the primary pathological change in the early stages.
- Selective neuronal death: Includes apoptosis, necrosis, and infarction, primarily involving gray matter.
- White matter injury: Specifically affects the parasagittal area; posterior cerebral white matter damage may also occur as a primary injury type in HIE, though it is less common.
- Hemorrhage: Intracerebral, subarachnoid, intraventricular, or primary brain parenchymal hemorrhage may occur.
Clinical Manifestations
Clinical symptoms of neonatal hypoxic-ischemic encephalopathy (HIE) vary depending on the neonate's age, the severity of the injury, and its duration. Neurological symptoms typically appear within 6–12 hours after birth, gradually worsen, peak at around 72 hours, and then start to improve. In cases of severe injury, deterioration or death often occurs within the first 72 hours.
Birth to 12 hours
The primary symptoms during this period are secondary to cerebral hemispheric suppression. Infants often exhibit difficulty in being aroused and display periodic breathing. Pupillary responses remain intact, and spontaneous eye movements may occur. Within 6–12 hours after birth, about half of the affected infants may present with hypotonia, tremors, or seizures. The Moro, grasp, suckling, and swallowing reflexes may be absent or suppressed. In more severe cases, seizures can occur as early as 2–3 hours after the asphyxic event.
12–24 hours
During this time, affected infants show increased irritability, and some may initiate seizures or exhibit apnea, tremors, and proximal limb weakness (upper limbs are more affected than lower limbs). The Moro reflex becomes hyperactive, crying becomes shrill and monotonic, and deep tendon reflexes (biceps, radial, knee, and Achilles reflexes) are heightened.
24–72 hours
In severely affected infants, the level of consciousness further deteriorates, leading to deep stupor or coma. Respiratory arrest commonly occurs following episodes of irregular breathing. Brainstem dysfunction is prevalent during this period, and most deaths in severely affected infants happen during this time frame.
Beyond 72 hours
Survivors at this stage typically show gradual improvement over days to weeks; however, some neurological abnormalities may persist. Infants may exhibit mild to moderate lethargy and feeding difficulties. Brainstem dysfunction is particularly pronounced in infants with selective neuronal necrosis in deep nuclei. Based on changes in consciousness, muscle tone, primitive reflexes, the presence of seizures, disease course, and prognosis, HIE is commonly classified into mild, moderate, and severe grades.
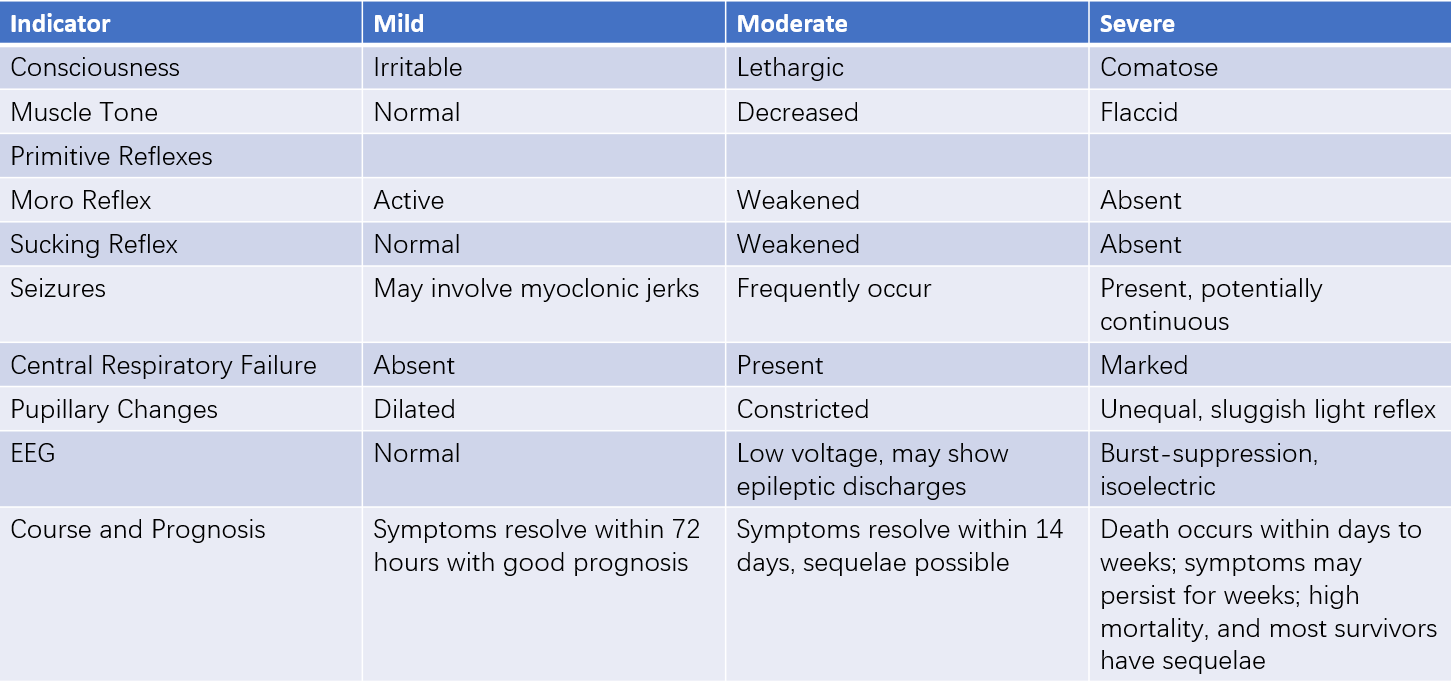
Table 1 Clinical grading of HIE
Auxiliary Examinations
Blood Gas Analysis
At birth, umbilical artery blood samples should be collected for blood gas analysis. A low pH indicates intrauterine hypoxia and the severity of acidosis, while BE and PaCO2 values help determine the nature of the acidosis. Umbilical artery samples and arterial blood samples within one hour after birth provide critical information for the diagnosis of HIE and should be thoroughly assessed.
Neuroimaging Examinations
Cranial Ultrasound
This is useful for detecting brain edema, basal nuclei and thalamus abnormalities, intraventricular or periventricular hemorrhage. However, it is less sensitive to parasagittal region injuries. Cranial ultrasound can be performed during the early stages of HIE (within 72 hours) and monitored dynamically over time.
CT
CT provides information on the extent and type of intracranial hemorrhage and offers some reference for brain edema, basal nuclei, and thalamus injuries. However, CT cannot be performed at the bedside, involves significant radiation, and is therefore used less frequently.
MRI
MRI offers excellent resolution for distinguishing between brain gray and white matter without radiation exposure. Axial, sagittal, and coronal views clearly display areas not easily visualized by ultrasound or CT, making MRI particularly sensitive for detecting parasagittal region injuries. MRI provides essential information for determining the type, extent, and severity of brain injury as well as predicting outcomes in term and preterm infants. Common sequences for HIE include T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and magnetic resonance spectroscopy (MRS). The American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) recommend performing early MRIs between 24 and 96 hours after birth, with follow-up scans at around 10 days of age (optimal window: 7–21 days). Early DWI may show decreased apparent diffusion coefficient (ADC) values in the thalamus and basal ganglia, while elevated lactate/N-acetylaspartate ratios on proton MRS are often associated with poor prognosis. Late-stage evaluation is more focused on T1WI and T2WI sequences, which are highly predictive of neurodevelopmental abnormalities when basal ganglia, thalamus, or the posterior limb of the internal capsule show abnormalities.
Neurophysiological Examinations
Full-Montage Video Electroencephalogram (EEG)
This is acutely performed to assess the severity of HIE, identify candidates for therapeutic hypothermia, detect seizures, and evaluate prognosis. EEG findings in HIE typically include delayed cortical activity (compared to gestational age), abnormal discharges, and background abnormalities (e.g., low voltage or burst suppression patterns). Continuous monitoring (or daily monitoring for 1–2 hours) is recommended until normalized EEG patterns are observed or by postnatal day 4.
Amplitude-Integrated EEG (aEEG)
This is a simplified version of conventional EEG that is convenient, cost-effective, and enables bedside monitoring of brain function in critically ill neonates. aEEG is useful for grading HIE severity and predicting prognosis. While it may miss some seizures, it can be used for seizure screening. Interpretation challenges may require complementary full-montage video EEG.
Evoked Potential Testing
Bedside testing of auditory evoked potentials, somatosensory evoked potentials, and visual evoked potentials can help assess the severity of HIE, prognosis, and potential abnormalities in visual and auditory pathways.
Other Examinations
Comprehensive evaluations of complete blood count (CBC), C-reactive protein (CRP), liver and kidney function, coagulation profile, electrolytes, and calcium-phosphorus-magnesium levels are necessary to assess the involvement of other organ systems. Blood cultures should be conducted if infection is suspected. For suspected genetic or metabolic disorders, blood ammonia levels and tandem mass spectrometry analysis of blood and urine samples should be performed.
Diagnosis
The current diagnostic criteria for neonatal hypoxic-ischemic encephalopathy (HIE) include the following:
- A clear history of abnormal obstetric events that could result in intrauterine fetal distress, along with significant signs of fetal distress, such as fetal heart rate below 100 beats per minute lasting for more than five minutes and/or Grade III meconium-stained amniotic fluid, or a significant history of asphyxia during delivery.
- Severe asphyxia at birth, defined as an Apgar score of ≤3 at 1 minute and ≤5 at 5 minutes after birth, combined with an umbilical artery blood gas pH of ≤7.00 at birth.
- Neurological symptoms appearing shortly after birth and persisting for more than 24 hours, including altered consciousness (hyperexcitability, lethargy, or coma), abnormalities in muscle tone (increased or decreased), and impaired primitive reflexes (weakened or absent suckling and Moro reflexes). Severe cases may present with seizures, brainstem symptoms (altered breathing patterns, abnormalities in pupil size, sluggish or absent light reflex), and increased cranial fontanel tension.
- Exclusion of seizures and brain injury caused by alternative conditions, such as electrolyte imbalances, isolated intracranial hemorrhage, obstetric trauma, intrauterine infections, genetic metabolic disorders, or other congenital conditions.
A definitive diagnosis can be made if all four criteria are met. Cases where the fourth criterion cannot yet be confirmed are considered provisional diagnoses. No standard diagnostic criteria currently exist for HIE in preterm infants.
Treatment
Supportive Therapy
Maintaining optimal ventilation is fundamental to supportive therapy, with a target partial pressure of arterial oxygen (PaO2) between 50–70 mmHg and normal levels of arterial carbon dioxide (PaCO2) and pH. The type of oxygen therapy is determined based on blood gas results.
Supporting adequate cerebral and systemic perfusion is a key measure for supportive therapy. Cerebral perfusion should be neither too low nor excessively high, and fluctuations should be avoided. Dopamine or dobutamine may be used to maintain blood pressure within the normal range to ensure sufficient and stable cerebral perfusion. Echocardiography plays an important role in managing circulatory function.
Blood glucose levels should remain within the normal range, as both hyperglycemia and hypoglycemia can exacerbate brain injury. Blood glucose should be closely monitored, especially during the first 24 hours.
Seizure Control
Seizures are a common symptom in severe HIE and controlling them can help reduce cerebral metabolic activity. Phenobarbital is the first-line treatment, with a loading dose of 20 mg/kg administered intravenously over 15–30 minutes. If seizures persist, an additional dose of 10–20 mg/kg can be given one hour later, followed by a maintenance dose of 3–5 mg/kg per day at 12–24 hours. Midazolam at a dose of 0.1–0.3 mg/kg may be added intravenously for refractory seizures.
Management of Cerebral Edema
Prevention and treatment of cerebral edema are based on avoiding excessive fluid administration. Accurate recording of input and output volumes guides adjustments in fluid intake. Total fluid volume on the first day is generally 60–80 ml/kg. Diuretics, steroids, and mannitol are not recommended.
Therapeutic Hypothermia
Therapeutic hypothermia involves artificially reducing the body temperature to 33–34°C to decrease energy consumption, reduce extracellular glutamate, and inhibit oxidative reactions, thereby protecting brain cells. This is currently the only treatment for neonatal HIE proven to be safe and effective, and it has been shown to reduce the rates of severe disability and mortality. Therapeutic hypothermia is indicated for term neonates with moderate to severe HIE and can be achieved using either whole-body or head cooling. The treatment window is within the first 6 hours of life, during the phase of secondary energy failure, with earlier initiation associated with better outcomes. Therapy typically lasts for 72 hours.
Other Treatments
Treatments such as recombinant human erythropoietin, melatonin, allopurinol, and stem cell therapy remain in the clinical trial stage. The combination of therapeutic hypothermia with other pharmacological interventions is also being explored in clinical research.
Post-Neonatal Period Therapy
Following stabilization, early initiation of cognitive and physical rehabilitation training may promote recovery of brain function and reduce the risk of long-term complications.
Prognosis and Prevention
The prognosis of HIE is associated with the Apgar score, the severity of the condition, and the accuracy and timeliness of resuscitation efforts. An Apgar score of ≤3 that persists for 15 minutes or longer, prolonged seizures, persistent consciousness abnormalities, brainstem symptoms lasting more than one week, and continued abnormal EEG findings are associated with high mortality. Survivors often experience varying degrees of motor or intellectual disabilities, epilepsy, and other sequelae. Strengthened perinatal care for mothers, widespread adoption of modern resuscitation techniques, and effective prevention and management of perinatal asphyxia are the primary strategies for preventing this condition.