A normal term infant refers to a live-born baby with a gestational age of 37 weeks ≤ gestational age < 42 weeks, a birth weight of 2,500 g ≤ birth weight ≤ 4,000 g, and no congenital anomalies or diseases. A preterm infant, also referred to as a "premature infant," is born before full term. According to the World Health Organization (WHO), the global prevalence of preterm birth ranges from 5% to 18%, with approximately 15 million preterm infants being born annually. The smaller the gestational age and the lower the birth weight of a preterm infant, the higher the mortality rate. The survival rates for infants receiving full treatment at gestational ages <32 weeks and <26 weeks are 95.4% and 65.6%, respectively. However, infants with a birth weight below 1,500 g account for more than 50% of neonatal deaths and more than 50% of disabled infants. This issue represents a significant challenge in the fields of medicine and ethics. Preventing preterm birth is therefore critical for reducing neonatal mortality and childhood disability rates. Factors such as maternal infections during pregnancy, smoking, alcohol consumption, drug abuse, trauma, genital abnormalities, excessive stress, and multiple pregnancies are the primary causes of preterm birth. Additionally, race and genetic predisposition are also linked to preterm birth.
External Characteristics of Normal Term Infants and Preterm Infants
The physical appearance of normal term infants and preterm infants differs based on their respective gestational ages. Gestational age assessment can be determined through evaluating physical characteristics and neurological maturity.
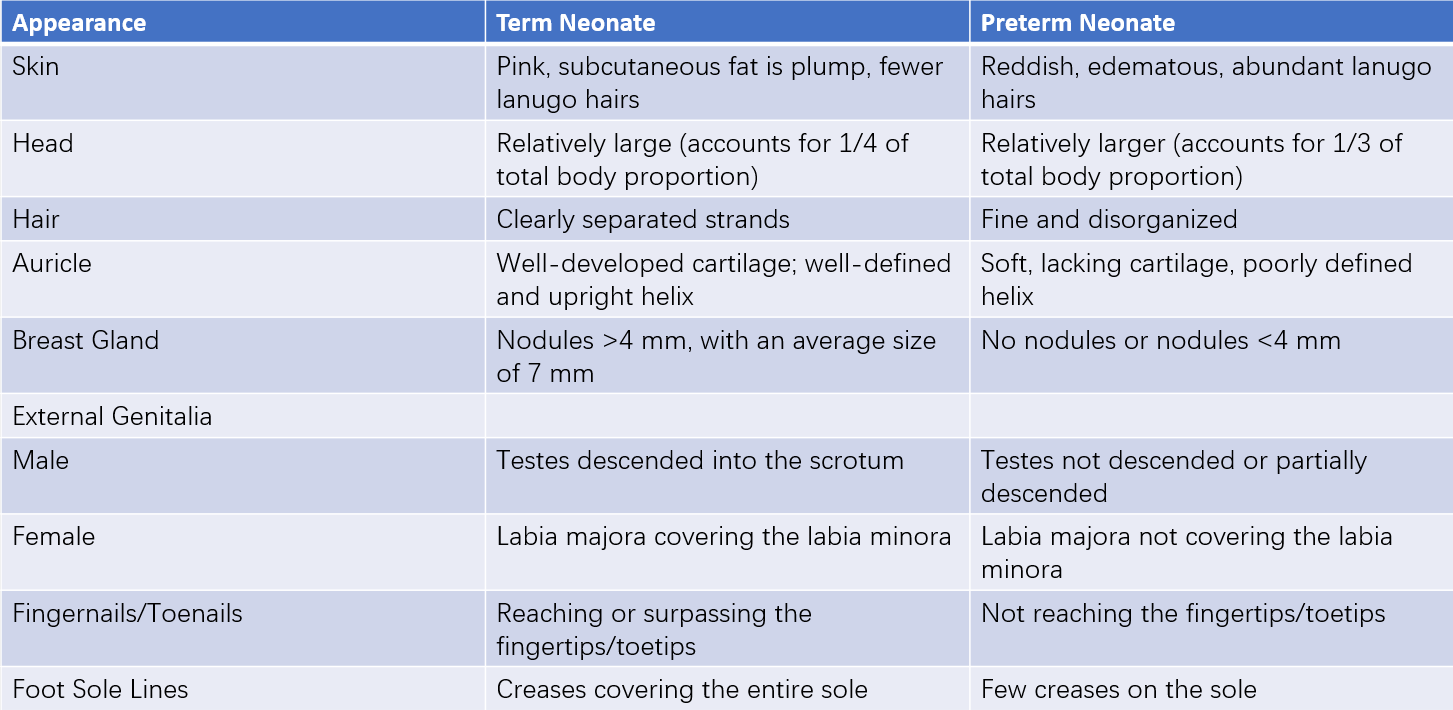
Table 1 Characteristics of term and preterm neonates
Physiological Characteristics of Normal Term Infants and Preterm Infants
Respiratory System
The lungs of a fetus are filled with fluid prior to birth. During natural delivery, the fetal alveolar epithelial sodium channel (ENaC) is rapidly upregulated under the influence of oxygen, catecholamines, glucocorticoids, and other hormones. This shift triggers a sudden change in alveolar epithelial cells from a secretion mode to an absorption mode, significantly reducing lung fluid. At full-term delivery, approximately 30–35 ml/kg of lung fluid is present. About one-third to one-half of this fluid is expelled through the mouth and nose due to the compression experienced during passage through the birth canal, while the remaining fluid is absorbed by capillaries and lymphatic vessels in the pulmonary interstitium after the onset of breathing. Absence of birth canal compression and the microenvironmental stimuli that promote lung fluid clearance in infants delivered via elective cesarean section may result in delayed fluid absorption, causing transient tachypnea of the newborn (TTN). Neonates typically have a faster respiratory rate of around 40 breaths/min at rest, and a rate exceeding 60 breaths/min is considered tachypnea, often indicating respiratory or other systemic conditions. The neonatal ribcage appears barrel-shaped, intercostal muscles are weak, and respiration relies primarily on diaphragmatic movement, demonstrated as abdominal breathing. Neonatal airways are narrow, mucosa is delicate, blood vessels are rich, and ciliary motion is inefficient, making neonates susceptible to airway obstruction, infections, respiratory distress, and feeding difficulties.
Respiratory function in preterm infants is immature due to several factors:
- Immature respiratory centers, resulting in reduced sensitivity to hypoxia and hypercapnia.
- Reduced production of carbonic anhydrase in red blood cells, leading to decreased production of carbon dioxide and less stimulation of the respiratory centers.
- Fewer alveoli, flattened cuboidal epithelial cells in respiratory mucosa, a greater distance between capillaries and alveoli, and reduced gas exchange efficiency.
- Underdeveloped respiratory muscles and a weaker cough reflex.
Preterm infants often exhibit shallow, rapid, and irregular breathing. Cyclic breathing patterns, where breathing pauses for 5–10 seconds followed by resumed respiration with no changes in heart rate, oxygen saturation, or cyanosis, are common. Apnea episodes, defined as airflow cessation ≥20 seconds accompanied by a heart rate below 100 beats/min, cyanosis, desaturation, and in severe cases, pallor and reduced muscle tone, are frequently observed in preterm infants. The shorter the gestational age, the higher the incidence of apnea, which typically manifests within the first 1–2 days after birth and resolves by a gestational age of 37 weeks.
Due to low surfactant levels in alveoli, preterm infants are at high risk for respiratory distress syndrome. Immature lung development, combined with high airway pressure, high lung volume, high oxygen levels, infections, and inflammatory damage, may lead to bronchopulmonary dysplasia (BPD), also known as chronic lung disease (CLD).
Circulatory System
Significant hemodynamic changes occur in the circulatory system after birth:
- The clamping of the umbilical cord terminates blood circulation between the placenta and umbilical vessels.
- Initiation of breathing and lung expansion decrease pulmonary vascular resistance and increase pulmonary blood flow.
- Increased blood flow to the left atrium and elevated systemic pressure occur.
- Functional closure of the foramen ovale occurs.
- Elevated oxygen partial pressure leads to functional closure of the ductus arteriosus.
These changes facilitate the transition from fetal circulation to adult circulation.
When severe pneumonia, acidosis, or hypoxemia is present, pulmonary vascular pressure may rise, reaching or exceeding systemic pressure. This can result in reopening of the foramen ovale and ductus arteriosus, producing right-to-left shunting, a condition referred to as persistent fetal circulation (PFC), now termed persistent pulmonary hypertension of the newborn (PPHN). Neonatal heart rate ranges from 90 to 160 beats/min, and in term infants, the average blood pressure is approximately 70/50 mmHg.
Preterm infants often present with elevated heart rates and lower blood pressure. Patent ductus arteriosus (PDA) may be observed in some preterm infants during the early postnatal period.
Digestive System
At birth, term infants typically exhibit a well-developed swallowing mechanism, although the lower esophageal sphincter remains relaxed, the stomach is positioned horizontally, and the pyloric sphincter is relatively strong. These features contribute to a tendency for regurgitation or even vomiting. The digestive tract has a relatively large surface area, thin walls, and heightened mucosal permeability, which facilitate the absorption of liquid nutrients and breast milk. However, these characteristics also allow toxins and incompletely digested substances to penetrate into the bloodstream, potentially causing toxicity or allergic reactions. Except for amylase, term infants are capable of producing sufficient digestive enzymes; therefore, starchy foods should generally be avoided during this early stage. Meconium, which consists of fetal intestinal secretions, bile, and swallowed amniotic fluid, is viscous and dark green. In term infants, meconium is generally passed within 24 hours, and its excretion is completed within 2–3 days. Failure to pass meconium within the first 24 hours requires evaluation for conditions such as imperforate anus or other gastrointestinal malformations. Insufficient levels and activity of uridine diphosphate glucuronosyltransferase in the liver are among the main causes of physiological jaundice. Additionally, impaired glucuronidation affects the metabolism of certain drugs, increasing the risk of drug toxicity.
Preterm infants often exhibit weaker sucking ability, an underdeveloped swallowing reflex, and reduced gastric capacity, which frequently result in feeding difficulties or aspiration of milk, potentially causing aspiration pneumonia. While the levels of digestive enzymes are comparable to those in term infants, bile acid secretion is limited, leading to reduced fat digestion and absorption. Complications such as necrotizing enterocolitis may arise due to adverse factors, including hypoxia, infection, or improper feeding. Meconium excretion is often delayed in preterm infants due to its reduced formation and weaker intestinal peristalsis. Immature liver function in preterm infants leads to more severe and prolonged physiological jaundice, with an increased risk of kernicterus. Additionally, limited hepatic synthesis of proteins and reduced glycogen reserves predispose preterm infants to conditions such as hypoproteinemia, edema, and hypoglycemia.
Urinary System
The renal structures in term infants are fully developed at birth, although their functionality remains immature. While the renal dilution capacity is comparable to that of adults, the glomerular filtration rate is low, and concentration capacity is limited. These characteristics reduce the ability of term infants to efficiently manage excess water and solutes, increasing the risk of edema. Most neonates begin urinating within the first 24 hours after birth, with some initiating urination within 48 hours. By the end of the first week, daily urine frequency in term infants may reach approximately 20 times.
Preterm infants exhibit even lower renal concentrating capacity and impaired tubular reabsorption of sodium due to reduced aldosterone responsiveness, leading to a higher risk of hyponatremia. A lower renal glucose threshold predisposes preterm infants to glucosuria. The renal tubular bicarbonate threshold and acid excretion capabilities are also significantly diminished. High levels of protein and casein in cow milk formulas may increase endogenous hydrogen ion production, which, when exceeding the tubular excretory capacity, results in late metabolic acidosis. Symptoms of late metabolic acidosis include pallor, lethargy, failure to gain weight, and metabolic acidosis. This condition has become rare with the widespread use of formula milk specifically designed for infants.
Hematologic System
At birth, hemoglobin levels in term infants average 170 g/L (140–200 g/L). Due to limited fluid intake and insensible water loss immediately after birth, hemoconcentration occurs, leading to increased hemoglobin levels, which generally peak within 24 hours and return to baseline levels by the end of the first week. Hemoglobin levels below 140 g/L in venous blood (or 20% higher levels in capillary blood) within the first week are defined as neonatal anemia. Fetal hemoglobin accounts for 70–80% at birth, decreases to 55% by 5 weeks of age, and is gradually replaced by adult hemoglobin. The reticulocyte percentage is 4–6% within the first 3 days after birth, rapidly decreases to 0.5–1.5% between days 4–7, and rises again to 2–8% by 4–6 weeks. Blood volume in term infants ranges from 85–100 ml/kg and is influenced by umbilical cord clamping timing. Delayed umbilical cord clamping for 1 minute can increase blood volume by approximately 35%. Leukocyte counts range from (15–20) × 109/L on the first day of life, decrease significantly by day three, and approximate infant values by day five. Neutrophils predominate initially, reach an equilibrium with lymphocytes by days 4–6, and are later surpassed by lymphocytes. Platelet counts in term infants are similar to those in adults. Due to limited fetal liver storage of vitamin K, coagulation factors II, VII, IX, and X exhibit reduced activities.
In preterm infants, blood volume ranges from 85–110 ml/kg. Nucleated red blood cells are abundant in peripheral blood. Leukocyte and platelet counts are slightly lower compared to term infants. By the end of the third week, many preterm infants exhibit transient eosinophilia, which persists for approximately two weeks. The combined effects of reduced erythropoietin production, lower innate iron stores, and rapidly expanding blood volume result in the earlier onset, prolonged duration, and increased severity of physiological anemia in preterm infants, with smaller gestational ages being more significantly affected.
Nervous System
At birth, the relative size of the neonatal head is large, with an average head circumference of 33–34 cm. Head growth slows at around 40 weeks of postnatal age, increasing by approximately 1.1 cm per month prior to this time. Brain sulci and gyri are not yet fully developed. The spinal cord is relatively elongated, with its terminal end located at the lower border of the L3–L4 vertebrae, making lumbar punctures typically performed at the L4–L5 intervertebral space. Cortical excitability in term infants is low, leading to prolonged sleep durations and wakeful periods lasting merely 2–3 hours per day. Cortical inhibition of subcortical centers is weak, and incomplete development of the corticospinal tract and striatum frequently results in uncoordinated and involuntary movements. Various transient primitive reflexes, such as the rooting reflex, sucking reflex, grasp reflex, and Moro reflex, are observable in term neonates. These reflexes typically disappear within several months postpartum. Abnormalities, such as weakened or absent primitive reflexes at birth or their persistence beyond the expected timeframe, may indicate neurological disorders or other abnormalities. Additionally, term infants may occasionally exhibit pathological reflexes typically seen in older children, such as Kernig's sign, Babinski's sign, and Chvostek's sign. The abdominal wall and cremasteric reflexes are unstable, with sporadic occurrences of ankle clonus.
Neurological maturity in preterm infants is directly correlated with gestational age. Lower gestational ages are associated with diminished or incomplete primitive reflexes.
Body Temperature
The thermoregulatory center in neonates remains underdeveloped. Subcutaneous fat is thin, and body surface area is relatively large. The keratinized layer of the skin is also thinner, making heat loss more pronounced, particularly in preterm infants. In response to cold, neonates do not exhibit shivering but rely on brown fat for thermogenic heat production via chemical metabolism. The environmental temperature postpartum is significantly lower than intrauterine conditions, leading to increased heat loss. Without proper warming measures, hypothermia, hypoxemia, hypoglycemia, metabolic acidosis, or cold injury may occur. The term "neutral temperature" or "neutral thermal environment" (NTE) refers to the environmental temperature at which the body maintains normal body temperature with minimal metabolic rate and oxygen consumption. The required neutral temperature differs based on birth weight and age, with lower birth weights and younger ages necessitating higher temperatures. Normal neonatal surface body temperature ranges from 36.0–36.5°C, while normal core (rectal) body temperature is between 36.5–37.5°C. Excess insensible water loss may increase energy expenditure, while optimal environmental humidity is between 50%–60%. Excessively high environmental temperatures, insufficient water intake, and ineffective heat dissipation can cause elevated body temperatures, potentially resulting in dehydration fever.
In preterm infants, the thermoregulatory center is even less mature. Limited brown fat reserves reduce heat production capacity, increasing susceptibility to hypothermia or cold injury in colder environments. Poor sweat gland development also predisposes preterm infants to hyperthermia in excessively warm conditions.

Table 2 Neutral temperatures for neonates with different birth weights
Energy and Fluid Metabolism
For neonates, basal energy expenditure is approximately 209 kJ/kg (50 kcal/kg), with total daily energy requirements estimated at 418–502 kJ/kg (100–120 kcal/kg). Preterm infants, due to weaker sucking ability and underdeveloped digestive function, may require parenteral nutrition in the first few weeks of life if these energy needs cannot be met.
Water content constitutes 70%–80% of a newborn's body weight and varies with birth weight and postnatal age. Lower birth weight and younger age are associated with higher water content. Consequently, neonatal water requirements depend on birth weight, gestational age, postnatal age, and clinical factors. On the first day after birth, water requirements are 60–100 ml/kg per day, increasing by 30 ml/kg daily until reaching 150–180 ml/kg per day. Neonates lose significant water postpartum, resulting in weight loss that reaches its lowest point (less than 10% of birth weight, or 15%–20% for preterm infants) by approximately one week of age. Birth weight is typically regained within around 10 days, a phenomenon known as physiological weight loss. The rate of weight recovery in preterm infants is slower compared to term infants. Following the onset of normal diuresis, fluid requirements increase from the third day of life. Neonates receiving intravenous nutrition require approximately 3 mmol sodium/(kg·d) and 2–3 mmol potassium/(kg·d).
Immune System
Both non-specific and specific immunity in neonates are immature. Fragile skin and mucosal membranes are easily damaged, while the umbilical stump remains open and located close to blood vessels, providing an entry point for bacteria into the bloodstream. Poor ciliary function in the respiratory tract, limited gastric acid and bile secretion, and reduced bactericidal ability further compromise the immune defense. Secretory immunoglobulin A (SIgA) deficiency also increases susceptibility to respiratory and gastrointestinal infections. Incomplete development of the blood-brain barrier makes bacterial meningitis more likely. Plasma complement levels are low, opsonin activity is reduced, and polymorphonuclear leukocytes exhibit deficient production, reserves, chemotaxis, and phagocytic function. These deficiencies are even more pronounced in preterm infants.
While immunoglobulin G (IgG) is transferred across the placenta, levels are gestational age-dependent, and smaller gestational ages correlate with lower IgG concentrations. Immunoglobulin A (IgA) and immunoglobulin M (IgM) are not transferred through the placenta, leaving neonates at greater risk for bacterial infections, particularly those caused by gram-negative bacilli. Antibody-mediated immune responses are weak or delayed, especially in response to polysaccharide vaccines and encapsulated bacteria. Underdeveloped T-cell immune function represents a major cause of neonatal immunodeficiency, which is more pronounced in preterm infants. Over time, T-cell function gradually matures with antigen exposure.
Common Physiological Conditions in Newborns
Physiological Jaundice
This frequently occurs in neonates.
Epstein Pearls and "Mantis Mouth"
Yellow-white, rice-grain-sized nodules may appear along the midline of the hard palate or on the gingivae. These nodules arise from the accumulation of epithelial cells or secretions from mucous glands and are commonly referred to as Epstein pearls. They typically resolve naturally within a few weeks. Bilateral fatty pads, known as "mantis mouth," are located in the cheeks and facilitate sucking. Both Epstein pearls and "mantis mouth" are normal phenomena and should remain undisturbed to avoid infection. In some cases, newborns may present with teeth, known as natal teeth, often located in the lower incisors or other areas; removal is usually unnecessary.
Breast Enlargement and Pseudomenstruation
Breast tissue enlargement, reaching the size of a soybean or walnut, may occur in neonates of both sexes within 4–7 days postpartum. This condition, which resolves within 2–3 weeks, is associated with maternal estrogen, progesterone, and prolactin persisting in the newborn's bloodstream immediately after birth. While maternal estrogen and progesterone levels decline rapidly postpartum, prolactin levels remain elevated, contributing to breast enlargement. Some neonates may even secrete small amounts of milk, and the area should not be pressed to avoid infection. Some female infants may experience minor vaginal bleeding or increased non-purulent secretions 5–7 days postpartum due to the abrupt withdrawal of maternal estrogen. These symptoms may last up to one week.
Erythema Toxicum Neonatorum
This condition manifests as polymorphic erythematous macules or papules of varying sizes on the head, trunk, or limbs within 1–2 days of life. The rash resolves spontaneously after 1–2 days.
Milia
Milia are small, yellow-white papules resembling millet grains, formed due to sebaceous gland accumulation on the nose, nasal alae, or face. They naturally disappear following desquamation.
Care for Term and Preterm Infants
Thermal Regulation
Newborns are immediately dried with pre-warmed towels after birth, followed by measures to maintain warmth, ensuring they remain within a neutral thermal environment. Term infants should ideally stay with their mother, practicing "kangaroo care." Preterm infants, especially those with a birth weight of less than 2,000 g or those experiencing hypothermia, are typically placed in incubators, with the neutral thermal environment adjusted based on gestational age, birth weight, and postnatal age. Humidification devices in incubators are prone to bacterial growth, so the water is replaced daily. In the absence of incubators, alternate warming methods, such as pre-warmed blankets, are used for neonates. Given the large surface area of the newborn's head, which leads to significant heat loss, velvet hats are commonly worn during colder seasons.
Nutrition
Term infants are breastfed within the first 30 minutes postpartum to stimulate lactation, with feeding on demand being encouraged. Infants without access to breast milk receive formula feeding every three hours, totaling seven to eight times daily. Milk volume is calculated based on energy requirements and an infant's tolerance, following gradual increments. Feeding adequacy is assessed by factors such as post-feeding calmness, absence of abdominal distension, and ideal weight gain (approximately 15–30 g/day for term infants, averaging 20 g/day), excluding periods of physiological weight loss.
Preterm infants are also breastfed as early as possible, taking into account their specific conditions. Preterm breast milk contains higher levels of protein, essential fatty acids, energy, minerals, trace elements, immune components like SIgA, and lactoferrin compared to milk from mothers of term infants, facilitating a faster return to birth weight. Infants with poor sucking ability, uncoordinated swallowing, or illness may be fed expressed breast milk via tube feeding. In the absence of breast milk, preterm formula is used temporarily. Feeding volume is individualized, with smaller volumes and shorter intervals required for infants of lower gestational ages and birth weights. Adjustments to feeding are based on factors such as abdominal distension, vomiting, gastric residuals (for tube-fed infants), and weight gain. Very low birth weight (VLBW) and extremely preterm infants may undergo trophic feeding, where small volumes of milk (10–20 ml/kg/day) are provided to initiate early feeding and promote intestinal development. If breastfeeding alone does not meet the energy requirements, parenteral nutrition is supplemented. Preterm infants discharged with corrected ages meeting appropriate-for-gestational-age (AGA) standards should prioritize breastfeeding. If unavailable, standard infant formula is used.
Preterm infants discharged with corrected ages falling below normal ranges benefit from breast milk fortifiers. Exclusive breastfeeding often fails to supply sufficient energy, protein, and minerals necessary for growth and development or achieving minimum intrauterine growth rates in low-birth-weight preterm infants. Exclusively breastfed extremely preterm infants may develop metabolic bone disease due to bone resorption being triggered to maintain normal serum calcium levels, occasionally resulting in fractures in severe cases. Breast milk fortifiers improve weight gain velocity and meet the expected nutritional requirements for preterm infants, including normalizing indicators such as nitrogen balance, protein nutritional status, calcium, phosphorus, alkaline phosphatase, urinary calcium, and urinary phosphorus. Fortifiers are typically introduced when milk intake reaches 50–100 ml/kg/day, starting with a half-dose and increasing to a full dose after 48 hours if well-tolerated. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommends the use of fortifiers until at least 40 weeks of corrected gestational age or, based on growth trends, continuing until 52 weeks of corrected gestational age. For infants without breast milk, preterm discharge formulas containing higher levels of protein, minerals, and trace elements are used until catch-up growth is achieved. Inadequate long-term nutrition can result in extrauterine growth retardation (EUGR), where postnatal weight, height, or head circumference falls below the 10th percentile for the corresponding gestational age. Overnutrition may also lead to long-term risks, such as insulin-resistant diabetes, dyslipidemia, and cardiovascular diseases.
Respiratory Management
Maintaining airway patency is critical, especially for preterm infants, who may benefit from a soft cushion placed under the shoulders when lying supine to avoid neck flexion. Hypoxemia is managed with oxygen supplementation, maintaining arterial oxygen partial pressure at 50–80 mmHg (50–70 mmHg for preterm infants) or transcutaneous oxygen saturation at 91%–95%. Routine oxygen supplementation in preterm infants is avoided to prevent retinopathy of prematurity (ROP) or bronchopulmonary dysplasia (BPD).
For apnea, interventions such as gentle stimulation of the soles or patting are effective in restoring breathing. Additionally, methylxanthines like caffeine citrate or aminophylline are administered, with caffeine citrate being preferred due to its higher safety profile and the absence of routine serum level monitoring requirements. An initial loading dose of 20 mg/kg is followed by a maintenance dose of 5 mg/kg daily, continuing until corrected ages of 34–35 weeks. Continuous positive airway pressure (CPAP) support is provided when necessary. Secondary apnea is managed by addressing the underlying cause.
Infection Prevention
Strict adherence to disinfection and isolation protocols is required among nursery staff. Handwashing is mandatory prior to infant contact, while sterile techniques are maintained during care and procedures. Staff or newborns with infectious diseases must be immediately isolated to avoid cross-infection. Overcrowding is avoided, and measures are taken to prevent air and milk product contamination.
Vitamins
Intramuscular injection of 0.5–1 mg vitamin K1 is administered after birth to prevent late-onset vitamin K deficiency.
Skin and Mucosal Care
Regular bathing maintains skin cleanliness. Healthy newborns bathe daily starting 24 hours postpartum, while the perineal region is cleansed after each bowel movement with warm water. Diapers are changed frequently to prevent diaper rash or perineal redness.
The umbilical stump is kept clean and dry, typically detaching within 3–7 days postpartum. If there is mucus or bleeding after detachment, povidone-iodine is used for disinfection or the stump is re-tied. Granulation tissue is treated with silver nitrate cauterization, while purulent infections necessitate hydrogen peroxide or povidone-iodine along with appropriate antibiotics when indicated.
The mucosa of the oral cavity is left undisturbed.
Clothing is loose, soft, and free of buttons, with absorbent and soft diapers being preferred.
Immunization
BCG Vaccine
This is administered on the third day of life using intradermal injection or skin scratching methods. Intradermal injection results in a 1 cm indurated red swelling within 2–3 weeks, eventually forming a small pustule and resolving into a scab that falls off, leaving a permanent circular scar. For skin scratching, red swelling develops within 1–2 weeks, with pustulation and scabbing occurring over 3–4 weeks, resolving in 1–2 months. Immunization is deferred for preterm infants, neonates with skin lesions, febrile illnesses, or suspected congenital immune deficiencies, as these conditions may cause systemic infections, posing life-threatening risks.
Hepatitis B Vaccine
This is administered within 24 hours of birth, at one month, and at six months with a recombinant yeast hepatitis B vaccine dose of 5 μg each time. Infants born to hepatitis B virus carrier mothers receive intramuscular injection of hepatitis B immunoglobulin (HBIG; 100–200 IU) within six hours postpartum, along with a 10 μg dose of recombinant yeast hepatitis B vaccine in a separate injection site.
Neonatal Screening
Postnatal screening for congenital metabolic disorders, including congenital hypothyroidism and phenylketonuria, is conducted.