Nutritional vitamin D deficiency rickets results from insufficient vitamin D in children, leading to disruptions in calcium and phosphorus metabolism, inadequate mineralization of the growth plate and bone matrix, and subsequent skeletal abnormalities. It is the most common cause of rickets, particularly affecting infants, especially younger infants, who are considered a high-risk group.
Physiological Functions and Metabolism of Vitamin D
Vitamin D is recognized as one of the most critical biological regulators of calcium homeostasis in the body.
Sources of Vitamin D
Vitamin D refers to a group of biologically active fat-soluble steroid derivatives, including vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is found in plants, while vitamin D3 is synthesized in human or animal skin from 7-dehydrocholesterol through photochemical action of ultraviolet light in sunlight. Vitamin D3 is the primary source of vitamin D in the body.
In infants and young children, there are three main sources of vitamin D:
- Maternal-Fetal Transfer: A fetus obtains vitamin D from the mother via the placenta. The storage of 25-(OH)D3 in the fetus can meet postnatal growth needs for a certain period.
- Dietary Intake: Breast milk contains low levels of vitamin D, but fortified formula milk and rice cereal now contain higher levels of vitamin D, making these fortified foods an adequate source for infants and young children.
- Cutaneous Synthesis via Sunlight: Skin synthesis is the primary source of vitamin D for humans. 7-dehydrocholesterol (7-DHC) in the skin acts as a precursor for vitamin D biosynthesis. Ultraviolet light (290–320 nm wavelength) in sunlight converts 7-DHC into cholecalciferol, the endogenous form of vitamin D3. The amount of vitamin D3 produced in the skin depends on the duration of sun exposure, ultraviolet wavelength, and the exposed surface area of the skin.
Transport of Vitamin D
Vitamin D2 from the diet is absorbed in the small intestine via the brush border with the aid of bile and then enters the lymphatic system. Vitamin D3 produced in the skin is directly absorbed into the bloodstream. Both vitamin D2 and D3 lack biological activity in the human body. After entering systemic circulation, they bind to vitamin D-binding protein (DBP) in the plasma and are transported to the liver. Vitamin D must undergo two hydroxylation reactions in the body to become biologically active. The first hydroxylation occurs in liver cells, forming 25-hydroxyvitamin D3 (25-(OH)D3), which is the major circulating form of vitamin D. 25-(OH)D3 in circulation binds to α-globulin and is transported to the kidneys, where a second hydroxylation occurs in the mitochondria of proximal renal tubular epithelial cells, catalyzed by 1-alpha hydroxylase, forming the highly biologically active 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3).
Physiological Functions of Vitamin D
The concentration of 25-(OH)D3 released into the bloodstream from the liver is relatively stable and serves as an indicator of vitamin D levels in the body. Though 25-(OH)D3 can mobilize calcium from bones into the blood, it has low antirachitic activity within physiological concentration ranges. Under normal conditions, circulating 1,25-(OH)2D3 primarily binds to DBP and exerts its biological effects on target cells. 1,25-(OH)2D3 acts as one of the principal hormones for maintaining calcium and phosphorus metabolism, exerting its antirachitic effects by acting on target organs (intestine, kidneys, and bones).
It promotes the synthesis of a specific calcium-binding protein (CaBP) in intestinal mucosal cells, increasing calcium absorption in the intestine, along with phosphorus absorption. In addition, 1,25-(OH)2D3 may directly facilitate phosphorus transport.
It enhances the reabsorption of calcium and phosphorus, especially phosphorus, in the proximal renal tubules, increasing serum phosphorus levels and supporting bone mineralization.
It mobilizes calcium from bone by working with parathyroid hormone (PTH) to stimulate osteoclast maturation, promoting bone resorption and the release of calcium salts into the blood. Simultaneously, it stimulates osteoblasts, maturing osteoid tissue and promoting calcium salt deposition.
Currently, it is understood that vitamin D's functions extend beyond bone and calcium-phosphorus metabolism. 1,25-(OH)2D3 is also involved in regulating the proliferation, differentiation, and apoptosis of various cells throughout the body, influencing neuromuscular function and the immune system.
Regulation of Vitamin D Metabolism
Autoregulation
Under normal conditions, vitamin D synthesis and secretion are self-regulated based on the body’s needs and the concentration of 25-(OH)D3 in the blood. When the level of 1,25-(OH)2D3 reaches a certain threshold, it inhibits the hydroxylation of 25-(OH)D3 in the liver as well as the hydroxylation of 1,25-(OH)2D3 in the kidneys.
Regulation by Serum Calcium, Phosphate, Parathyroid Hormone (PTH), and Calcitonin
The production of 1,25-(OH)2D3 in the kidneys is indirectly regulated by serum calcium levels. In cases of hypocalcemia, PTH secretion increases, stimulating the synthesis of more 1,25-(OH)2D3 in the kidneys. PTH and 1,25-(OH)2D3 act on bone tissue to enhance osteoclast activity and reduce osteoblast activity, thereby increasing bone resorption and releasing calcium into the bloodstream to maintain physiological function. Conversely, during hypercalcemia, calcitonin (CT) is secreted, inhibiting renal hydroxylation of 1,25-(OH)2D3. Serum phosphate levels also modulate 1,25-(OH)2D3 synthesis, with hypophosphatemia stimulating its production and hyperphosphatemia suppressing it.
Etiology
Perinatal Vitamin D Deficiency
Inadequate maternal vitamin D status during pregnancy, particularly in the later stages, can lead to insufficient vitamin D storage in the fetus. Factors such as severe maternal malnutrition, liver and kidney diseases, chronic diarrhea, preterm birth, and multiple pregnancies are common causes of this insufficiency.
Limited Sunlight Exposure
Various factors influencing ultraviolet (UV) exposure, such as geographic latitude, season, air pollution, high-rise buildings blocking sunlight, limited outdoor activities, clothing (which reduces skin exposure), and UV protection measures, affect vitamin D synthesis.
Rapid Growth and Increased Demand
The rapid growth and development during early infancy increase the demand for vitamin D. Limited vitamin D storage in the body may not suffice to meet these needs, especially in preterm infants and twins.
Dietary Vitamin D Deficiency
Natural food sources of vitamin D are limited, which can result in insufficient dietary intake.
Impact of Diseases and Medications
Gastrointestinal or hepatobiliary diseases, such as infantile cholestasis and chronic diarrhea, may reduce vitamin D absorption. Severe liver or kidney damage may impair the hydroxylation of vitamin D, leading to insufficient production of 1,25-(OH)2D3 and resulting in rickets. Long-term use of anticonvulsant drugs, such as phenytoin and phenobarbital, can stimulate the activity of hepatic microsomal oxidase systems, accelerating the breakdown of vitamin D and 25-(OH)D3 into inactive metabolites. Glucocorticoids may antagonize the calcium-transporting effects of vitamin D.
Pathogenesis
Vitamin D deficiency rickets can be understood as skeletal damage caused by the body's efforts to maintain blood calcium levels. Severe and prolonged vitamin D deficiency impairs calcium and phosphorus absorption in the intestine, resulting in hypocalcemia. This condition leads to increased secretion of parathyroid hormone (PTH), which mobilizes calcium from the bones to stabilize blood calcium levels at or near normal. However, PTH concurrently suppresses renal tubular reabsorption of phosphorus, further exacerbating calcium and phosphorus metabolic disturbances. The resultant decline in the calcium-phosphorus product in extracellular fluid disrupts calcium deposition in bone tissue.
Inadequate calcium and phosphorus concentrations in the extracellular fluid impair normal chondrocyte proliferation, differentiation, and apoptosis. Disruption of calcified cartilage architecture leads to the disappearance of the calcification line and irregular, disorganized epiphyseal plates. Bone matrix mineralization is impaired, causing compensatory proliferation of osteoblasts, increased secretion of alkaline phosphatase, and osteoid accumulation at the metaphysis. This leads to metaphyseal thickening and outward protrusions, forming "rachitic rosaries" and "wrist or ankle bracelets." Subperiosteal bone mineralization is incomplete, resulting in abnormal bone formation, cortical thinning, osteoporosis, and bending of weight-bearing bones. Skull ossification is disrupted, leading to cranial softening and the "square-shaped skull" appearance. These changes manifest clinically as a series of rickets-related symptoms and alterations in biochemical parameters.
Clinical Manifestations
The clinical presentation of vitamin D deficiency rickets varies with age due to differences in the skeletal growth rate. The disease course is classified into four stages:
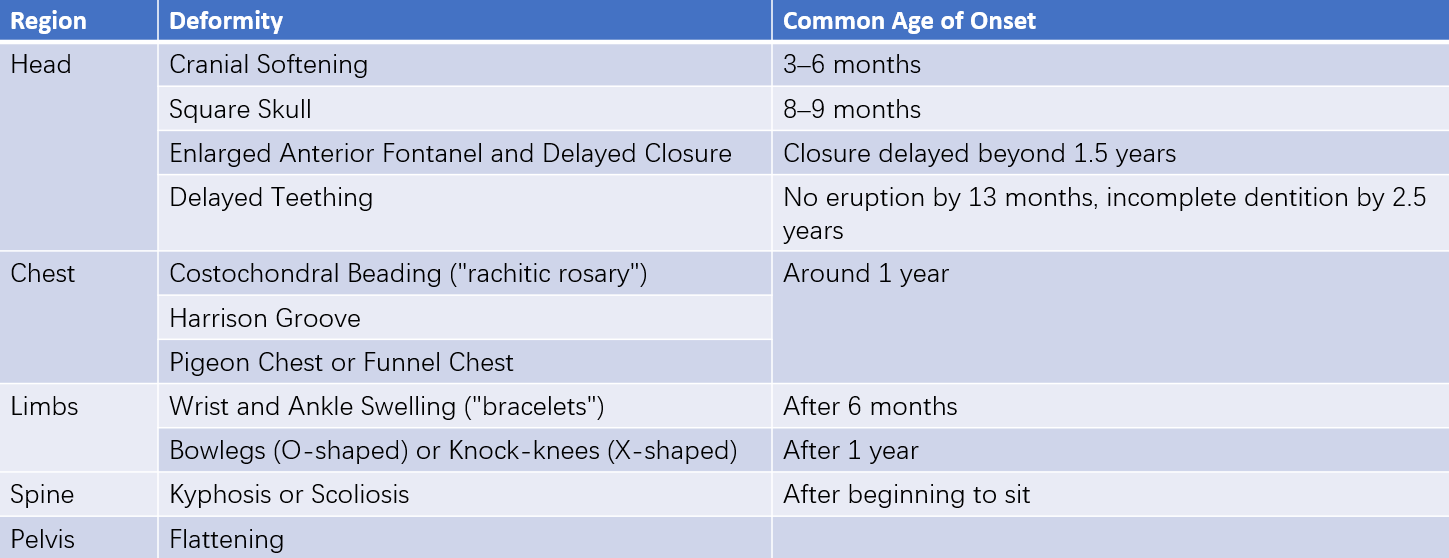
Table 1 Skeletal deformities in the active stage of nutritional vitamin D deficiency rickets and their common ages of onset
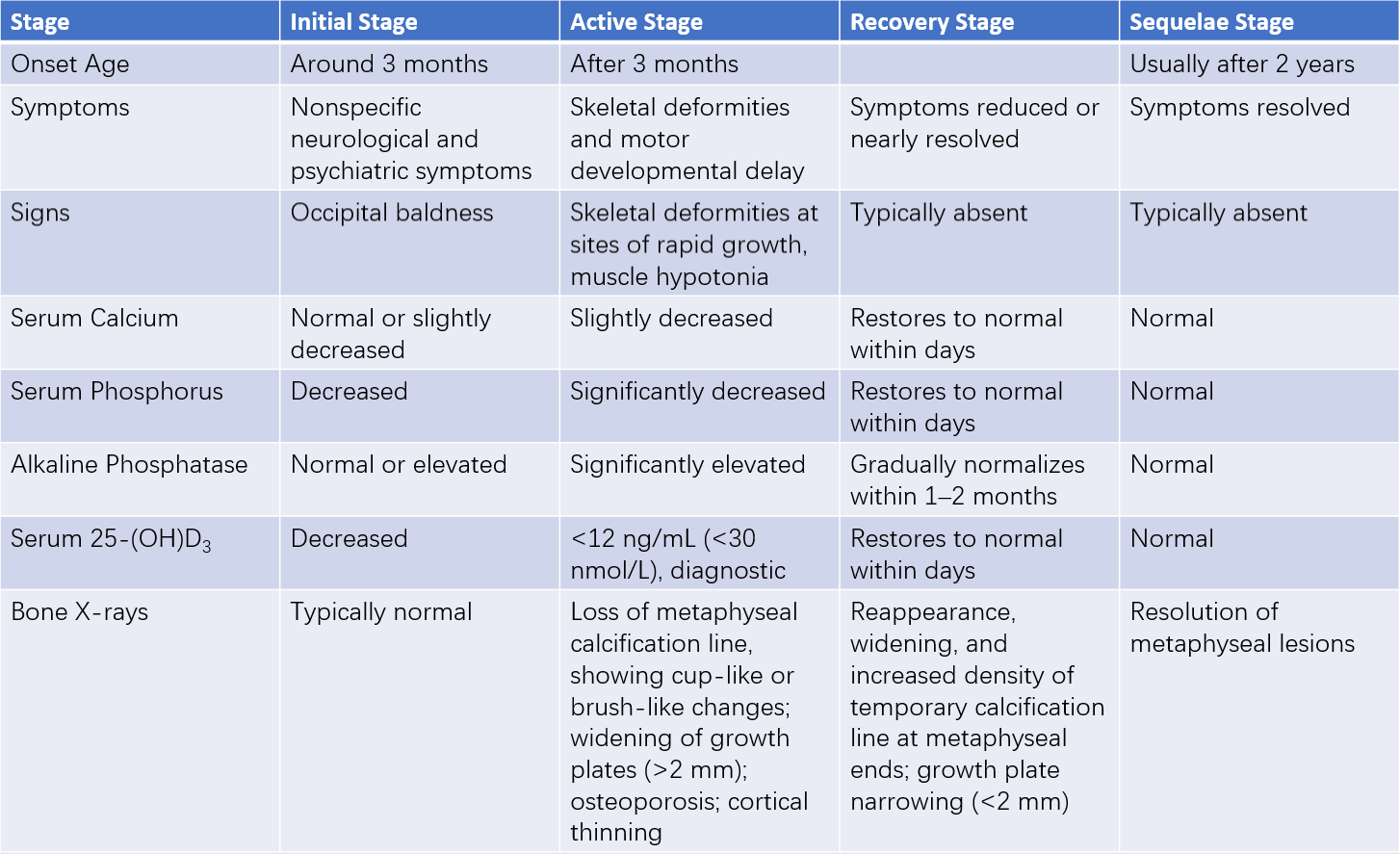
Table 2 Characteristics of the four stages of nutritional vitamin D deficiency rickets
Initial (Early) Stage
This stage is most common in infants under six months of age, particularly those younger than three months. Clinical signs primarily include increased neuromuscular excitability, such as irritability, restlessness, and excessive sweating leading to scalp irritation and head rubbing. These nonspecific symptoms are considered early diagnostic clues. Serum 25-(OH)D3 levels decrease, PTH levels increase, transient hypocalcemia occurs, and serum phosphorus levels drop, while alkaline phosphatase levels are normal or slightly elevated. Bone changes are typically absent or minimal on X-rays, showing either normal ossification or mild calcification line blurring.
Active (Exacerbation) Stage
If untreated, early vitamin D deficiency progresses, leading to PTH overactivity and characteristic skeletal changes due to calcium-phosphorus metabolism abnormalities. The most rapidly growing skeletal sites in the specific age group are the primary areas affected:
Skull
In infants under six months, rickets primarily manifests as skull changes. The edges of the anterior fontanel become softer, and the skull bones thin. On palpation, the occipital or parietal bones may demonstrate a "ping-pong ball" sensation when compressed with mild pressure. (This can also occur near sutures in normal infants.) Beyond six months, skull softening diminishes even if the disease progresses. The central areas of the frontal and parietal bones thicken, forming a "boxy" or "square-shaped" skull by 7–8 months of age, with an enlarged head circumference.
Thorax
The metaphyseal accumulation of osteoid causes bulging at the costochondral junctions, palpable as nodular protrusions along the ribcage. These bead-like structures, most notable from the 7th to 10th ribs, are termed "rachitic rosary." Around the age of one year, children may exhibit sternal and adjacent cartilage protrusion, resulting in a "pigeon chest" deformity. In severe cases, diaphragmatic attachments to ribs generate inward rib depression, producing a horizontal groove at the lower thoracic margin, known as Harrison's groove.
Limbs
Rounded, enlarged metaphyseal structures may develop at the wrists and ankles, referred to as "wrist" or "ankle bracelets." Bone softening, along with joint and muscle laxity, may lead to weight-bearing bones, such as the femur, tibia, and fibula, bowing under pressure after the child begins standing or walking. This bowing can result in severe genu varum (bowlegs, "O-shaped" deformity) or genu valgum (knock-knees, "X-shaped" deformity), sometimes producing a "K-shaped" appearance.
Other Findings
After children learn to sit or stand, ligament laxity may cause spinal deformities. Severe hypophosphatemia alters muscle glucose metabolism, leading to generalized muscle weakness, hypotonia, and reduced muscle strength.
During this stage, serum calcium levels may only be slightly reduced, but other biochemical abnormalities become more pronounced. X-rays reveal the disappearance of the calcification line, metaphyseal brush-like and cup-like changes, epiphyseal growth plate widening (>2 mm), cortical thinning of bones, osteoporosis, bowing of shafts, and greenstick fractures, often without clinical symptoms.
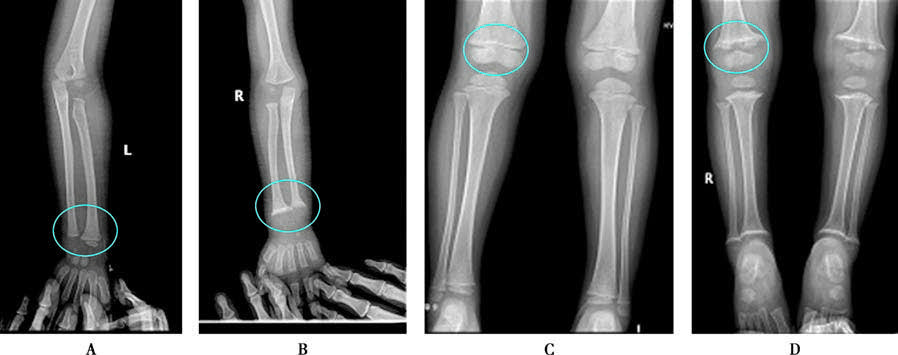
Figure 1 X-ray changes in rickets
A. Normal wrist; B. Wrist in rickets; C. Normal knee; D. Knee in rickets.
Recovery Stage
With treatment and sunlight exposure, clinical symptoms and physical signs from any previous stage gradually subside or disappear. Serum calcium and phosphorus levels normalize, and alkaline phosphatase levels usually return to normal within 1–2 months. X-ray changes improve 2–3 weeks after treatment, showing irregular calcification lines, which later become dense and thick. The growth plate narrows (<2 mm) and ultimately returns to normal morphology.
Sequelae Stage
This stage is more common in children aged two years or older. Residual skeletal deformities from severe rickets during infancy persist to varying extents. Clinical symptoms are absent, serum biochemical markers are normal, and X-rays show the resolution of metaphyseal lesions.
Diagnosis
A correct diagnosis requires an evaluation of the etiology of vitamin D deficiency, clinical manifestations, biochemical markers, and skeletal X-ray findings. Early symptoms of increased neuromuscular excitability, such as excessive sweating and irritability, are nonspecific and provide limited diagnostic accuracy when based solely on clinical presentation. Skeletal changes are reliable but not sensitive enough. Serum 25-(OH)D3 levels serve as the most reliable diagnostic criterion. A serum 25-(OH)D3 concentration of less than 12 ng/mL (<30 nmol/L) indicates vitamin D deficiency, while a level of ≥20 ng/mL (≥50 nmol/L) reflects vitamin D sufficiency. Values between these thresholds suggest a potential subclinical deficiency.
Differential Diagnosis
Differentiation from Skeletal Symptoms of Rickets
Skeletal changes associated with rickets need to be differentiated from other conditions such as mucopolysaccharidosis, achondroplasia, and hydrocephalus.
Differentiation of Rickets-Like Symptoms with Distinct Etiologies
Hypophosphatemic Vitamin D-Resistant Rickets
This condition is predominantly X-linked but can also present as autosomal dominant or recessive inheritance. It results from primary defects in renal tubular phosphate reabsorption and intestinal phosphate absorption. Symptoms generally appear after age one, with active rickets features persisting beyond age two or three. Serum calcium is typically normal, serum phosphorus is significantly reduced, and urinary phosphate excretion is increased. Lack of response to routine rickets therapy necessitates differentiation from this condition.
Distal Renal Tubular Acidosis
This disorder stems from inadequate hydrogen ion secretion in distal tubules, leading to significant urinary losses of sodium, potassium, and calcium. Secondary hyperparathyroidism and bone decalcification subsequently result in rickets-like manifestations. Affected children present with pronounced skeletal deformities, short stature, metabolic acidosis, polyuria, alkaline urine, hypocalcemia, hypophosphatemia, hypokalemia, and elevated serum ammonia levels.
Vitamin D-Dependent Rickets
This autosomal recessive condition is categorized into two subtypes. Type I results from a deficiency in renal 1-alpha hydroxylase, impairing the conversion of 25-(OH)D3 to 1,25-(OH)2D3, with normal 25-(OH)D3 concentrations. Type II involves defects in target organ receptors for 1,25-(OH)2D3, leading to elevated serum 1,25-(OH)2D3 levels. Children with type I may present with aminoaciduria, whereas a distinctive feature of type II is alopecia.
Renal Rickets
Chronic kidney dysfunction, whether congenital or acquired, disrupts calcium and phosphorus metabolism, resulting in hypocalcemia, hyperphosphatemia, secondary hyperparathyroidism, and generalized bone demineralization. Skeletal changes resembling rickets gradually become evident, often accompanied by growth retardation.
Hepatic Rickets: Since the liver is the site of the first hydroxylation step of vitamin D, conditions such as acute hepatitis, congenital extrahepatic biliary atresia, or other liver diseases may lower circulating 25-(OH)D3 levels. This reduction can result in hypocalcemia, seizures, and rickets-like manifestations.
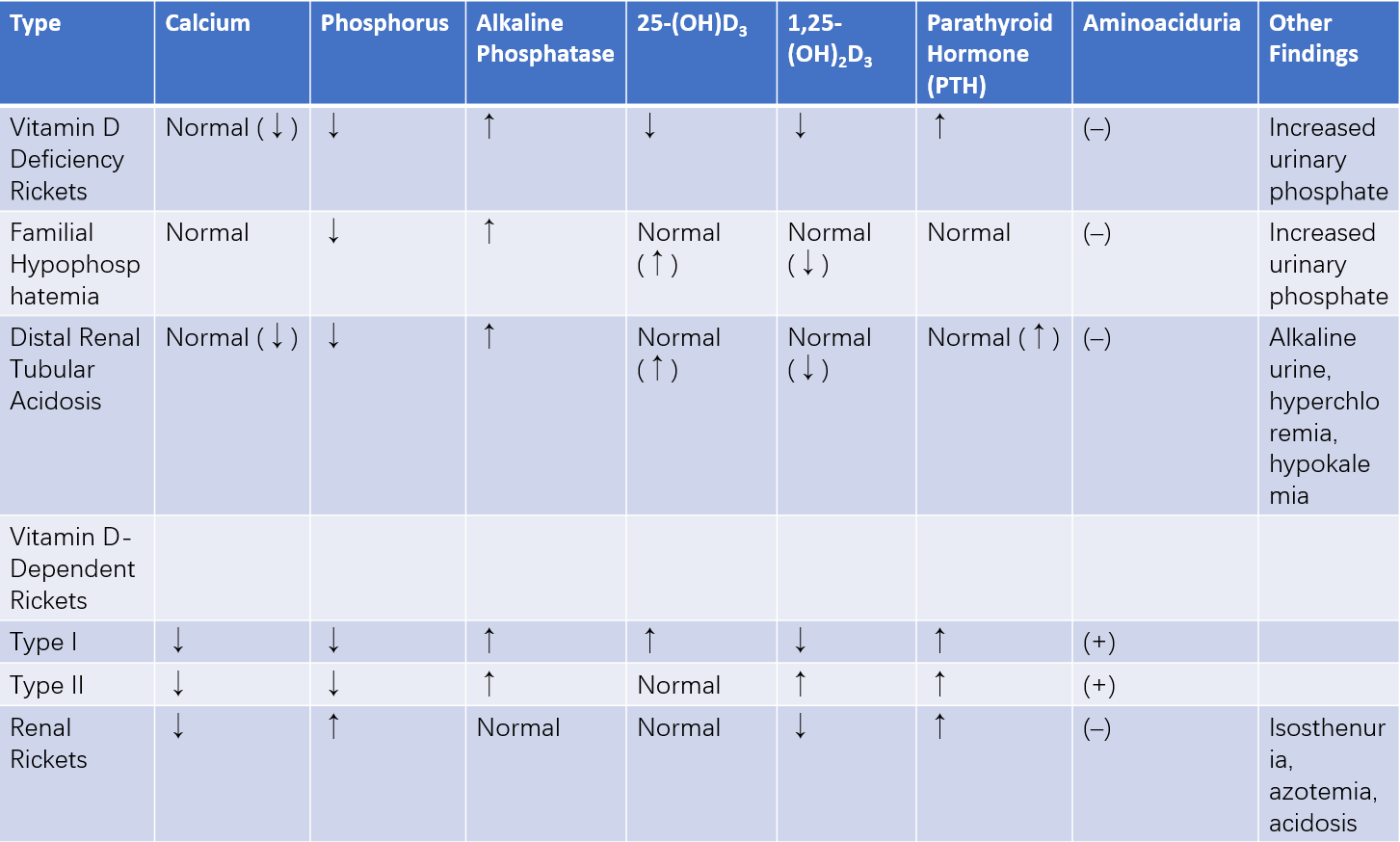
Table 3 Laboratory findings in different types of rickets (active stage)
Treatment
The aim of treatment is to control active symptoms and prevent skeletal deformities.
General Management
Providing good care, maintaining a balanced diet, and ensuring regular sunlight exposure are critical. For infants under six months, direct sunlight exposure should be avoided to prevent skin damage.
Vitamin D Therapy
High-dose vitamin D therapy is not generally recommended. Oral administration is preferred, with typical doses during the active stage being 2,000–4,000 IU/day (50–100 μg/day) for one month, followed by a maintenance dose of 400–800 IU/day. If oral administration is difficult (e.g., in cases of absorption issues or diarrhea), a single high-dose injection of 150,000–300,000 IU (3.75–7.5 mg) of vitamin D can be administered intramuscularly, followed by a maintenance dose of 400–800 IU/day after one month. If there is no improvement in symptoms, signs, or laboratory findings after one month of treatment, other diseases should be considered and differential diagnosis performed.
Other Treatments
Calcium Supplementation
Administering appropriate amounts of calcium alongside vitamin D can alleviate symptoms and promote bone development. Dietary adjustments to increase calcium intake from food sources are also advised.
Micronutrient Supplementation
Vitamin D-deficient rickets is often accompanied by reduced levels of zinc and iron. Timely and adequate supplementation of trace elements can benefit bone growth.
Orthopedic Treatment
Severe skeletal deformities may require corrective surgery.
Prevention
Efforts to prevent vitamin D deficiency and vitamin D-deficiency rickets should begin in the perinatal period and focus on infants and young children, continuing through adolescence.
Prevention During Fetal Development
Pregnant women should engage in regular outdoor activities for sunlight exposure.
Diets rich in vitamin D, calcium, phosphorus, and protein are encouraged.
Pregnancy complications should be treated promptly, and hypocalcemia or osteomalacia in expectant mothers should be actively managed.
Vitamin D supplementation at 800–1,000 IU/day and calcium intake can be initiated during the third trimester.
Prevention in Healthy Children Aged 0–18 Years
Outdoor Activity
Regular sunlight exposure provides a simple and effective method of preventing vitamin D deficiency and rickets. Gradual increases in sun-exposed skin area and duration of exposure are recommended, averaging 1–2 hours per day outdoors. For infants under six months, direct sunlight exposure should be avoided to prevent skin damage.
Vitamin D Supplementation
Regardless of feeding method, all infants require 400 IU/day of vitamin D. Children over 12 months require at least 600 IU/day.
Supplementation for High-Risk Groups
Preterm infants, low birth weight infants, and twins should receive 800–1,000 IU/day of vitamin D starting immediately after birth for three months, followed by 400–800 IU/day.