Characteristics of Fluid Balance in Children
Body fluids constitute an essential component of the human body, and maintaining their physiological balance is a vital condition for sustaining life. The dynamic equilibrium of water, electrolytes, acid-base status, and osmotic pressure in body fluids depends on the normal regulatory functions of systems such as the nervous, endocrine, respiratory, and particularly the renal systems. Due to specific physiological characteristics in children—such as a higher proportion of body weight comprised of body fluids, immature organ development, and less effective mechanisms for regulating fluid balance—they are more susceptible to imbalances in water, electrolytes, and acid-base homeostasis.
Total Body Fluid and Its Distribution
Body fluids are distributed among plasma, interstitial spaces, and intracellular compartments. Plasma and interstitial fluids together constitute the extracellular fluid. Younger children have a relatively higher total body fluid volume, primarily due to a greater proportion of interstitial fluid, while the proportions of plasma and intracellular fluid are similar to those observed in adults.
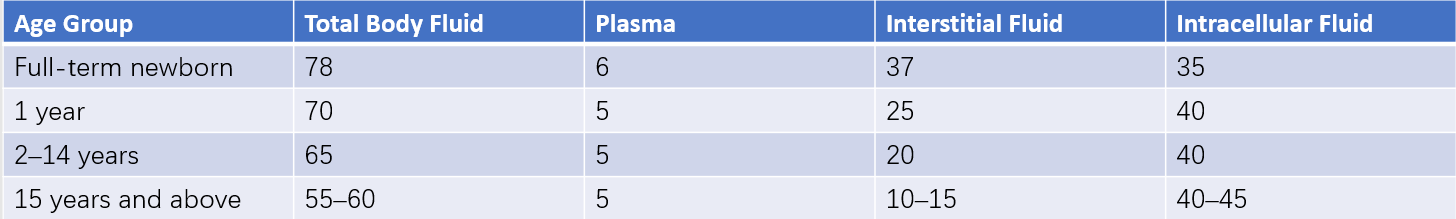
Table 1 Percentage of body weight composed of body fluids at different ages (Unit: %)
Electrolyte Composition of Body Fluids
The electrolyte composition of intracellular and extracellular fluids differs significantly. The electrolyte content of extracellular fluid can be precisely measured through blood plasma analysis. The main cations in plasma are Na+, K+, Ca2+, and Mg2+, with Na+ accounting for more than 90% of total cations in this compartment, playing a dominant role in maintaining the osmotic pressure of extracellular fluid. Major anions in plasma include Cl-, HCO3-, and proteins. The total charge of these anions relative to cations results in unmeasured anions (UA), which primarily consist of inorganic sulfur and phosphate, as well as organic acids such as lactate and ketone bodies. In interstitial fluid, the electrolyte composition is similar to plasma, except that Ca2+ levels are about half those in plasma. Intracellular fluid electrolyte composition varies across tissues and is more difficult to measure. Predominant cations include K+, Ca2+, Mg2+, and Na+, with K+ constituting 78% of the total. Dominant anions include proteins, HCO3-, HPO42-, and Cl-.
Metabolic Characteristics of Water in Children
Although the daily intake of water and electrolytes in healthy children may fluctuate considerably, their internal balance remains relatively stable, with water intake roughly equaling water loss.
Physiological Water Requirements
Water requirements are influenced by factors such as metabolic rate, caloric intake, food composition, renal solute excretion, insensible water loss, physical activity, and environmental temperature. Children generally have higher water needs than adults due to their rapid growth and development, higher levels of physical activity, faster metabolism, greater caloric and protein intake, and higher renal solute excretion. Additionally, children have a larger surface area-to-weight ratio and a faster respiratory rate, both of which contribute to increased insensible water loss compared to adults. Water retention during tissue growth may further increase the need for fluid intake. When calculated by body weight, younger children require more water per day.

Table 2 Daily water requirements for children
Water Excretion
Water is primarily excreted via the kidneys (urine), with additional losses occurring through insensible water loss from the skin and lungs, as well as through the gastrointestinal tract (feces). Insensible water loss, which is often overlooked, can account for significant water loss in preterm infants, with up to one-quarter of their daily water loss occurring through evaporation from the skin and lungs. Factors such as lower maturity at birth, larger surface area, faster respiratory rate, elevated body temperature, higher ambient temperature, lower relative humidity, and greater activity levels can increase insensible water loss.
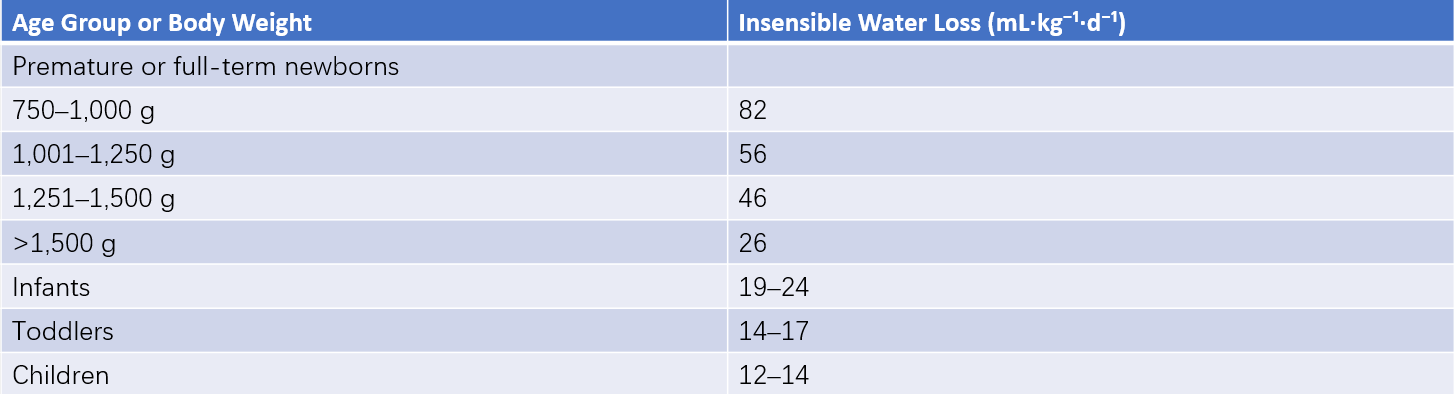
Table 3 Daily insensible water loss in children
The rate of water excretion in children is higher than in adults. The smaller the child, the higher the relative water exchange rate. In infants, the daily exchange of water is approximately one-half of their extracellular fluid volume, whereas in adults, it is only about one-seventh. As a result, infants are 3–4 times faster than adults in terms of water exchange rates. Due to an impaired tolerance for dehydration, infants are more vulnerable to water deficits in pathological conditions where water intake is insufficient, while fluid losses persist. Limited renal concentrating ability further predisposes infants to dehydration.
Regulation of Water Balance
The kidneys are the primary regulators controlling extracellular fluid volume and composition. Renal solute loads, including urea and salts (primarily sodium), require adequate urine volume for excretion. Water excretion by the kidneys is closely related to the secretion of antidiuretic hormone (ADH) and the responsiveness of renal tubular epithelial cells to ADH. The normal plasma osmolarity threshold for ADH secretion is 280 mOsm/L, with plasma osmolarity changes of as little as 1–2% capable of influencing ADH secretion. When fluid loss reaches 8% or more of total body fluid, ADH secretion increases significantly, exhibiting an exponential rise during severe dehydration.
The mechanisms regulating fluid balance in children are relatively immature. Under normal circumstances, renal concentration and dilution functions adjust water excretion based on fluid intake. When renal function is adequate, increased water intake leads to higher urine output, whereas reduced water intake or additional fluid losses (e.g., excessive sweating, vomiting, diarrhea) cause urine output to decrease. In younger children, these renal concentration and dilution capacities are less developed. For neonates and young infants, the reabsorptive capacity of renal tubules is immature, with a maximum urinary concentrating ability producing an osmolarity of approximately 700 mOsm/L (specific gravity of 1.020). This means that 1.0–2.0 mL of water is required to excrete 1 mmol of solute. In contrast, adults can achieve a urinary concentrating ability of up to 1,400 mOsm/L (specific gravity of 1.035), requiring only 0.7 mL of water per mmol of solute. Consequently, children excrete more water for the same solute load, resulting in relatively higher urine volume.
When water intake is inadequate or water losses are increased, the limited concentrating ability of the kidneys may lead to the retention of metabolic waste products and hyperosmolar dehydration. On the other hand, while newborns can dilute urine to 50–100 mOsm/L (specific gravity of 1.003) like adults after the first week of life, their low glomerular filtration rate limits the rate of water excretion. Excessive water intake may, therefore, result in edema and hyponatremia. Furthermore, the ability of the kidneys to excrete sodium, acid, and ammonia is also less developed in younger children, increasing the likelihood of hypernatremia and acidosis.
Disorders of Water and Electrolyte Metabolism
Dehydration
Dehydration refers to a reduction in the total body fluid volume, especially the extracellular fluid volume, due to insufficient water intake or excessive water loss. This condition is often accompanied by the loss of sodium, potassium, and other electrolytes.
Severity of Dehydration
The severity of dehydration depends on the rate and extent of water and electrolyte loss, commonly expressed as the percentage of body weight lost. If recent body weight records are unavailable, the percentage of body weight loss can often be estimated based on physical examination and patient history. Clinical signs such as the degree of sunken fontanel or eye sockets, skin elasticity, circulatory status, and urine output are used for comprehensive assessment. Dehydration is classified into three levels of severity:
- Mild dehydration: Indicates a loss of 3–5% of body weight or approximately 30–50 mL/kg of body fluid.
- Moderate dehydration: Indicates a loss of 5–10% of body weight or approximately 50–100 mL/kg of body fluid.
- Severe dehydration: Indicates a loss of more than 10% of body weight or approximately 100–120 mL/kg of body fluid.
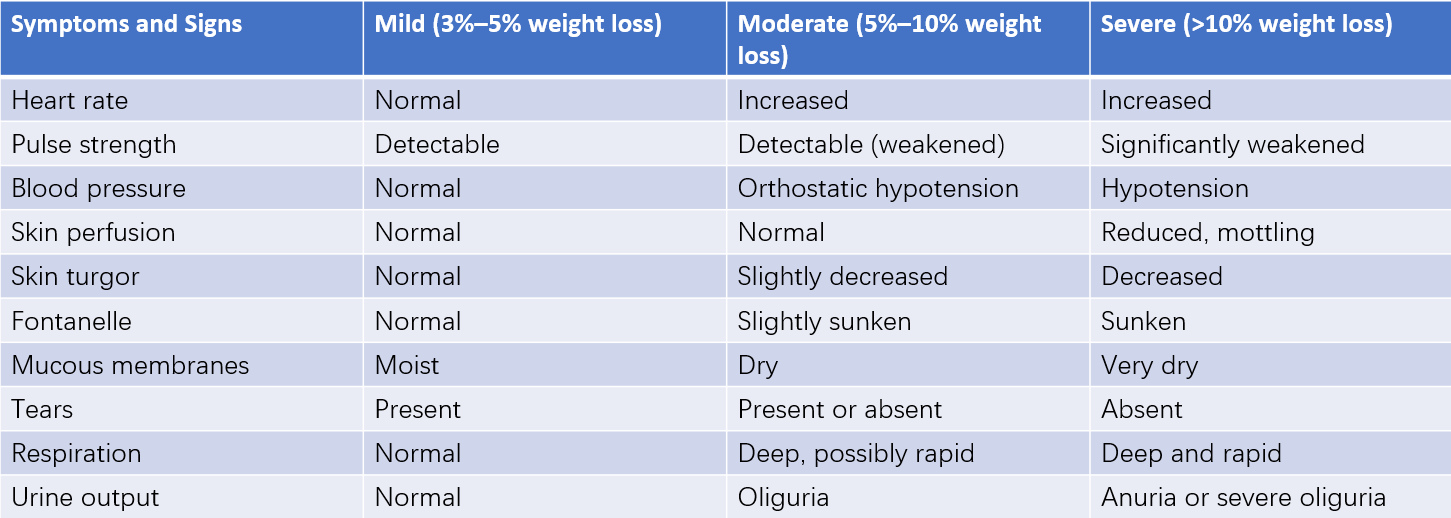
Table 4 Symptoms and signs associated with different degrees of dehydration
Clinical signs of moderate and severe dehydration often overlap, making it difficult to precisely calculate fluid loss per unit of body weight.
Types of Dehydration
The type of dehydration often reflects the relative loss of water and electrolytes. Clinical evaluation is primarily based on serum sodium concentration and plasma osmolarity levels.
Isotonic Dehydration
Serum sodium concentration ranges from 130–150 mmol/L, indicating proportional losses of water and electrolytes. Plasma osmolarity remains normal, and the lost fluid is mainly extracellular fluid. This type of dehydration is commonly observed in cases of acute diarrhea, vomiting, gastrointestinal fluid drainage, intestinal fistulas, or short-term starvation. Because there is no osmotic gradient between the intracellular and extracellular compartments, intracellular fluid volume remains unchanged. Clinical manifestations primarily depend on the degree of extracellular fluid loss.
Hypotonic Dehydration
Serum sodium concentration is less than 130 mmol/L, indicating greater electrolyte loss compared to water loss, leading to decreased plasma osmolarity. This type of dehydration often occurs after several days of diarrhea when water intake is normal but sodium intake is insufficient. It is also observed in malnutrition with chronic diarrhea, chronic kidney diseases, congestive heart failure treated with long-term sodium restriction and diuretic use, and patients with extensive burns.
In hypotonic dehydration, the extracellular fluid becomes hypotonic, causing water to move into the intracellular compartment. This leads to further reductions in extracellular fluid volume, more pronounced reductions in effective circulating blood volume, and intracellular edema. Compared to other types of dehydration, hypotonic dehydration manifests more severe clinical features even with equivalent water loss. Symptoms may include early onset of blood pressure reduction, decreased urine output, and shock. Severe hyponatremia can cause cerebral edema, leading to neurological symptoms such as headache, irritability, lethargy, coma, or seizures.
Hypertonic Dehydration
Serum sodium concentration exceeds 150 mmol/L, indicating greater water loss than electrolyte loss, with increased plasma osmolarity. The lost fluid is predominantly intracellular fluid. This type of dehydration is commonly associated with diarrhea accompanied by high fever, increased insensible water loss without adequate water supplementation (e.g., in cases of coma, fever, rapid respiration, phototherapy, infrared radiation for warmth, or prematurity), excessive intake of isotonic or hypertonic fluids via oral or intravenous routes, diabetes insipidus of central or renal origin, or excessive use of diuretics.
In hypertonic dehydration, the extracellular fluid becomes hypertonic, causing water to shift from the intracellular to the extracellular compartment. This leads to a reduction in intracellular fluid volume while partially compensating for extracellular fluid loss. For an equivalent volume of water loss, the clinical signs of hypertonic dehydration tend to be less severe compared to other types. Common symptoms include thirst, high fever, and irritability, with less pronounced circulatory impairment, and skin that often feels warm and dough-like. Neurological symptoms are prominent, including increased muscle tone, heightened reflexes, or even seizures.
Isotonic dehydration is the most common type observed clinically, followed by hypotonic dehydration, with hypertonic dehydration being relatively rare. It should be noted that the degree of dehydration is often overestimated in children with severe malnutrition. Sunken eye sockets, often recognized by parents, are one of the earliest clinical signs to improve following fluid therapy.
Potassium Metabolism Disorders
Potassium is predominantly found within cells, with an intracellular concentration of approximately 150 mmol/L. Normal serum potassium is maintained between 3.5–5.0 mmol/L and plays a critical role in regulating various cellular functions.
Hypokalemia
Hypokalemia refers to a serum potassium concentration of less than 3.5 mmol/L.
Etiology
Several primary causes contribute to hypokalemia:
- Inadequate potassium intake.
- Excessive gastrointestinal potassium loss, such as through vomiting, diarrhea, prolonged drainage, or frequent enemas without timely potassium supplementation.
- Excessive renal potassium excretion, caused by factors such as the use of potassium-wasting diuretics (loop diuretics, thiazides, carbonic anhydrase inhibitors), corticosteroids, β-adrenergic agonists, theophylline, or other drugs. Chronic kidney diseases associated with potassium loss (e.g., renal tubular acidosis, Bartter syndrome, Liddle syndrome), endocrinological disorders resulting in excess adrenal corticosteroid secretion (e.g., Cushing syndrome, primary hyperaldosteronism), diabetic ketoacidosis, hyperthyroidism, and hypomagnesemia can also result in hypokalemia.
- Abnormal distribution of potassium within the body. During the treatment of dehydration with acidosis, potassium loss may increase through the urine. Additionally, as acidosis is corrected, potassium shifts into cells. Processes such as glycogen synthesis, familial periodic paralysis, alkalosis from various causes, and the use of insulin can lead to potassium moving rapidly from extracellular fluid into cells, resulting in hypokalemia.
Clinical Manifestations
The clinical manifestations of hypokalemia depend not only on the serum potassium concentration but also on the rate at which hypokalemia develops. Slow-onset hypokalemia may lead to severe potassium depletion without pronounced symptoms. Major clinical symptoms fall into three categories:
- Decreased neuromuscular excitability, presenting as lethargy, muscle weakness (e.g., nausea, vomiting, abdominal distention, intestinal paralysis, respiratory muscle weakness or paralysis, flaccid paralysis), and diminished or absent tendon reflexes (e.g., reduced or absent knee and abdominal wall reflexes).
- Cardiovascular impairment, presenting as reduced myocardial contractility or cardiac enlargement. Symptoms may include muffled heart sounds, arrhythmias, reduced blood pressure, and possibly heart failure. Electrocardiographic changes may show flattened, broad T waves, the presence of U waves, prolonged QT intervals, inverted T waves, and ST-segment depression.
- Renal impairment, such as reduced renal concentrating ability leading to polyuria. Increased renal tubular secretion of H⁺ and bicarbonate reabsorption, along with reduced chloride absorption, may cause hypokalemic hypochloremic alkalosis. Chronic hypokalemia can also lead to nephron sclerosis, interstitial fibrosis, and reduced secretion of growth hormone.
Management of Hypokalemia
The primary disease must be treated alongside potassium supplementation:
For mild hypokalemia, oral potassium chloride is administered at a dose of 200–300 mg/kg per day.
For severe hypokalemia, intravenous potassium supplementation is required, with doses of 4–6 mmol/kg per day. The infusion rate and concentration must be calculated precisely because rapid potassium infusion, even in cases of severe hypokalemia, can pose risks such as fatal arrhythmias. In cases of renal dysfunction without urine output (anuria), potassium excretion is impaired, and potassium supplementation can lead to hyperkalemia; urinary output must be confirmed before initiating potassium administration (bladder retention or urine output within the previous 6 hours may be considered as evidence of urine production). The concentration of intravenous potassium should not exceed 0.3% (0.15–0.2% in neonates), and the total infusion duration should be no less than 6–8 hours per day. Serum potassium levels should be dynamically monitored during supplementation, and cardiac monitoring should be performed if conditions allow. Due to the slow recovery of intracellular potassium, hypokalemia treatment must often be continued for 4–6 days or longer. Potassium supplementation can be transitioned from intravenous to oral administration as the condition improves.
Hyperkalemia
Hyperkalemia refers to a serum potassium concentration above 5.5 mmol/L.
Etiology
The primary causes of hyperkalemia fall into three categories:
- Reduced renal potassium excretion, seen in conditions such as kidney failure, hyperkalemic renal tubular acidosis, adrenal insufficiency, and prolonged use of potassium-sparing diuretics.
- Excessive potassium intake through intravenous or oral routes, such as rapid or excessive potassium infusion during fluid therapy or transfusion of stored blood.
- Abnormal potassium distribution, such as potassium shifting from the intracellular to the extracellular compartment due to severe hemolysis, hypoxia, shock, metabolic acidosis, or severe crush injuries.
Clinical Manifestations
Hyperkalemia primarily presents with effects on the cardiovascular and neuromuscular systems:
- Electrocardiographic abnormalities and arrhythmias, resulting from the depolarizing effect of potassium on myocardial cell membranes. The cardiac conduction system is one of the earliest structures affected, with electrocardiographic changes often preceding clinical symptoms. Manifestations may include slowed and irregular heart rate, ventricular premature beats, ventricular fibrillation, and even cardiac arrest. Common electrocardiographic findings include peaked T waves, prolonged PR intervals, flattened P waves, widened QRS complexes, ST-segment depression, atrioventricular block, ventricular fibrillation, and cardiac arrest.
- Reduced neuromuscular excitability, resulting in symptoms such as lethargy, drowsiness, paresthesia in the hands and feet, reduced or absent tendon reflexes, flaccid paralysis, urinary retention, and, in severe cases, respiratory paralysis. The release of acetylcholine may also cause nausea, vomiting, and abdominal pain.
Management of Hyperkalemia
Treatment aims focus on two key objectives: (1) prevention of fatal arrhythmias, and (2) removal of excess potassium from the body. The underlying disease must be treated, potassium-containing drugs and foods should be discontinued, and adequate caloric intake should be ensured to prevent endogenous protein breakdown and subsequent potassium release. For serum potassium levels above 6 mmol/L, electrocardiographic monitoring is essential to evaluate the risk of arrhythmias.
There are three primary therapeutic approaches:
- Stimulating potassium uptake into cells to reduce serum potassium levels. Sodium bicarbonate (5%) can be administered intravenously at 3–5 mL/kg. Glucose with insulin can also be used (0.5–1 g of glucose/kg, with 1 unit of insulin administered for every 3–4 g of glucose). β₂-adrenergic agonists, such as salbutamol (5 μg/kg via intravenous administration over 15 minutes or 2.5–5 mg via nebulization), may effectively lower serum potassium levels for 2–4 hours.
- Counteracting the toxic effects of hyperkalemia on the heart. Calcium preparations can stabilize myocardial cell membranes and prevent arrhythmias. Calcium gluconate (10%) at 0.5 mL/kg can be administered via slow intravenous infusion over several minutes, with concurrent electrocardiographic monitoring. This approach is temporary as it does not significantly reduce total body potassium.
- Removing excess potassium from the body through measures such as ion-exchange resins (e.g., sodium polystyrene sulfonate), hemodialysis or peritoneal dialysis, or continuous blood purification (CBP). These methods often achieve significant results.
Acid-Base Balance Disorders
The body continuously produces acidic and basic substances during metabolism. Acid-base balance refers to the maintenance of a specific concentration of hydrogen ions (H+) in bodily fluids to keep the blood pH of children within a normal range of 7.35–7.45, ensuring proper metabolic and physiological function. The regulation of acid-base balance primarily depends on two mechanisms:
- Buffer systems.
- Regulation by the lungs and kidneys.
The buffer systems in the body include volatile and fixed acids. Volatile acids are primarily represented by dissolved CO2 in the plasma, which allows for rapid and powerful regulatory mechanisms for acid-base balance. Fixed acids primarily involve the most significant buffering pair in the blood: bicarbonate/carbonic acid (HCO3-/H2CO3). The pH of the extracellular fluid is primarily determined by the ratio of HCO3- to H2CO3 in the blood. Under normal conditions, the HCO3-/H2CO3 ratio is maintained at 20:1. When certain factors alter this ratio or when compensatory mechanisms are insufficient, bodily pH deviates from the normal range of 7.35–7.45, leading to acid-base imbalance.
The lungs contribute to acid-base regulation by modulating alveolar ventilation to control CO2 elimination, while the kidneys regulate acid-base balance by selectively reabsorbing or excreting acids and bases in the urine. The lungs act more quickly than the kidneys, adjusting pH within minutes, whereas the compensatory response of the kidneys requires hours or even days. However, both systems have limitations. Disorders of the respiratory system that reduce or enhance CO2 elimination can lead to acid-base imbalance, manifesting as respiratory acidosis or respiratory alkalosis. Metabolic disturbances that produce or deplete H2CO3 in the blood plasma can result in metabolic acidosis or metabolic alkalosis.
When acid-base imbalance occurs, the body can compensate by regulating HCO3-/H2CO3 levels to maintain a 20:1 ratio, keeping pH within the normal range, a condition termed compensated metabolic/respiratory acidosis (or alkalosis). If the ratio cannot be maintained and pH falls below or rises above the normal range, it is referred to as uncompensated metabolic/respiratory acidosis (or alkalosis). Acid-base imbalances are commonly classified as simple types (respiratory acidosis, respiratory alkalosis, metabolic acidosis, or metabolic alkalosis), though mixed types may also occur.
Metabolic Acidosis
Metabolic acidosis is the most common type of acid-base imbalance in clinical practice. It results from one of two possibilities:
- Excessive acid production in the extracellular fluid.
- Excessive loss of bicarbonate in the extracellular fluid.
Excessive acid production is commonly associated with conditions such as ketoacidosis, an increase in phosphoric and sulfuric acids in kidney failure, and increased lactic acid production during tissue hypoxia. Excessive bicarbonate loss occurs through the kidneys or small intestine, as seen in prolonged diarrhea or drainage of small intestinal fistulas.
The primary buffering component in metabolic acidosis is bicarbonate. Compensatory hyperventilation leads to increased CO2 elimination, which may lower PaCO2 and partially improve pH. However, pH rarely fully normalizes through respiratory compensation alone (partial compensation). Full compensation depends on the kidneys producing more acidic urine, restoring bicarbonate levels in the blood. Ultimately, acid-base balance can be restored to normal only when both kidneys and lungs are able to regulate together.
Based on the anion gap (AG) value, metabolic acidosis can be classified into two types:
- Normal AG metabolic acidosis (8–16 mmol/L).
- Elevated AG metabolic acidosis (>16 mmol/L).
Reduced AG metabolic acidosis occurs less frequently in clinical practice.
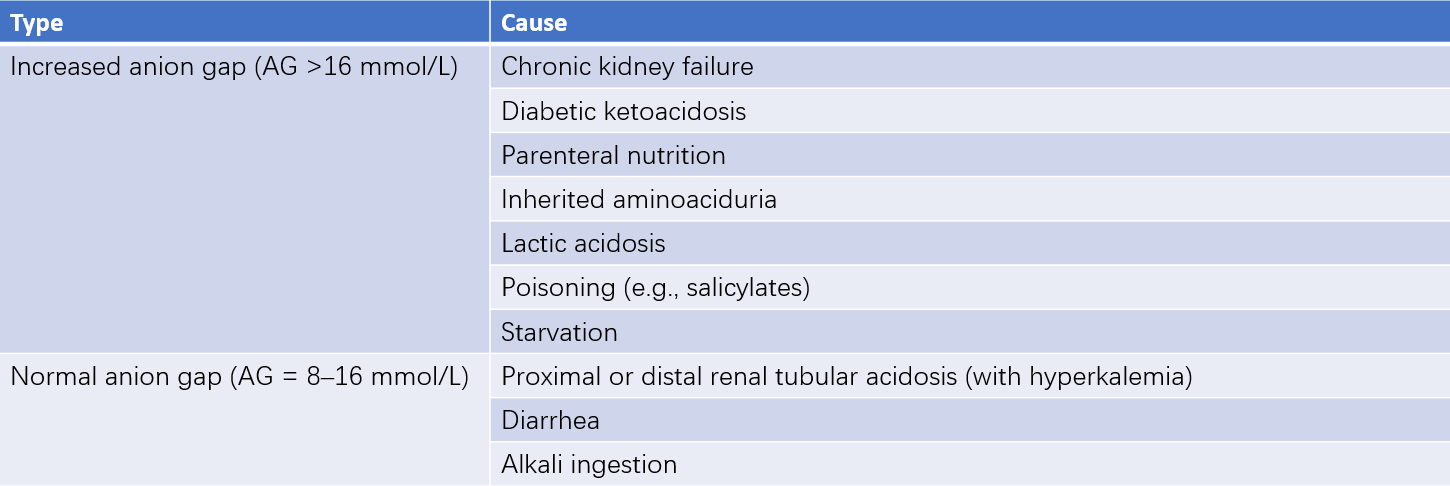
Table 5 Common causes of metabolic acidosis with increased or normal anion gap
Normal AG metabolic acidosis is primarily caused by bicarbonate loss, characterized by a normal AG, elevated blood chloride (referred to as hyperchloremic metabolic acidosis), and reduced HCO3- concentration. This type is commonly caused by excessive gastrointestinal bicarbonate loss (e.g., diarrhea) or impaired renal bicarbonate reabsorption due to renal tubular acidosis. It may also occur with the use of carbonic anhydrase inhibitors (e.g., acetazolamide), aldosterone antagonists, or excessive intake of acidic chloride salts such as calcium chloride and magnesium chloride.
Elevated AG metabolic acidosis is primarily caused by increased acid production (e.g., lactic acid or ketone bodies). It is often observed in conditions such as diabetic ketoacidosis, starvation-induced ketosis, or salicylate poisoning.
Clinical Manifestations
Mild metabolic acidosis may present with subtle symptoms, while more severe cases typically manifest as lethargy or restlessness, deep and rapid respiration, facial or lip redness, abdominal pain, vomiting, drowsiness, and coma. During acidosis, cellular H+/K+ exchange may increase extracellular potassium levels, which can lead to arrhythmias and heart failure. Elevated plasma calcium levels due to acidosis may drop following correction, potentially causing tetany in patients with preexisting hypocalcemia. Neonates and young infants exhibit poor respiratory compensation, and their respiratory changes during acidosis may be atypical, instead presenting as lethargy, feeding rejection, and pallor.
Management Principles
Treatment of underlying conditions such as hypoxia, tissue hypo-perfusion, or diarrhea is essential.
Alkalizing agents, such as sodium bicarbonate or sodium lactate, are used to increase alkaline reserves and neutralize H+. Mild metabolic acidosis may resolve spontaneously with appropriate treatment of underlying causes without alkalizing agents. The use of alkalizing agents is generally recommended for pH <7.30.
The required dose of alkaline solutions (in mmol) can be calculated using the formula:
Dose (mmol) = |-BE| x 0.3 x body weight (kg)
where BE (base excess) is derived from blood gas analysis. Larger negative BE values indicate greater deficits.
For 5% sodium bicarbonate, which contains 0.6 mmol of solute per mL, the required volume (in mL) can be calculated as:
Volume (mL) = |-BE| x 0.5 x body weight (kg)
Typically, 5% sodium bicarbonate is diluted to a 1.4% solution for infusion. Half of the calculated dose is administered initially, and blood gas levels are reassessed to adjust dosing. Sodium bicarbonate is not recommended in cases of ventilation dysfunction, as CO2 retention may exacerbate acidosis. Sodium lactate should also be avoided in neonates, hypoxia, shock, and liver dysfunction.
Correction of acidosis may cause intracellular potassium movement, leading to decreased serum potassium levels, and reductions in free calcium. Potassium and calcium supplementation should be administered promptly when needed.
Metabolic Alkalosis
Metabolic alkalosis results from the loss of hydrogen ions (H+) or the accumulation of bicarbonate (HCO3-). Five main causes are identified:
- Excessive hydrogen ion loss, such as severe vomiting or gastric drainage, leading to the loss of hydrogen and chloride. This is most commonly seen in congenital hypertrophic pyloric stenosis.
- Excessive intake or administration of bicarbonate-containing substances.
- Increased bicarbonate reabsorption by the kidneys due to hypokalemia, seen in conditions such as primary hyperaldosteronism or Cushing's syndrome.
- Renal compensatory mechanisms during respiratory acidosis, in which the kidney secretes hydrogen and increases bicarbonate reabsorption to compensate for acidosis. Following the correction of PaCO2 via mechanical ventilation, bicarbonate levels in the plasma may remain elevated, leading to metabolic alkalosis.
- Decreased extracellular fluid volume, coupled with increased bicarbonate reabsorption in the proximal renal tubule.
- During metabolic alkalosis, respiratory suppression may occur as a compensatory mechanism to reduce changes in blood pH, resulting in a slight increase in PaCO2 levels. However, the extent of respiratory compensation is limited due to the risk of hypoxia, which can, in turn, stimulate respiration. The kidneys may excrete bicarbonate to lower blood pH, which can result in alkaline urine (urine pH may reach 8.5–9.0). In cases of concurrent hypokalemia and hypovolemia, correction is necessary because these conditions make metabolic alkalosis difficult to manage otherwise.
Clinical Manifestations
Metabolic alkalosis lacks characteristic symptoms. Mild cases often present no obvious symptoms, while severe cases may result in respiratory suppression and lethargy. Hypocalcemia due to alkalosis may precipitate convulsions, and clinical signs of hypokalemia may also appear. Arterial blood gas analysis typically reveals elevated plasma pH, PaCO2, and HCO3- concentrations, with low chloride and potassium levels being common. In typical cases, urine is alkaline, but with severe hypokalemia, urine pH may be low.
Management Principles
These involve:
- Addressing the underlying cause.
- Discontinuing alkaline medications and correct fluid and electrolyte imbalances.
- Administering intravenous normal saline.
- Use of intravenous ammonium chloride in severe cases.
Simultaneous correction of accompanying hyponatremia, hypokalemia, and hypochloremia is essential, as these can hinder the resolution of metabolic alkalosis.
Respiratory Acidosis
Respiratory acidosis is caused by ventilation impairment that leads to CO3 retention and an increase in H2CO3 levels. Common causes include:
- Diseases of the respiratory system, such as pneumonia, emphysema, airway obstruction (e.g., foreign bodies, thick secretions, amniotic blockage, laryngeal spasms, or edema), bronchial asthma, pulmonary edema, atelectasis, pulmonary collapse, or respiratory distress syndrome (RDS). Limited respiration due to chest diseases, such as pneumothorax, pleural effusion, trauma, or surgery, is another cause.
- Neuromuscular diseases, such as myasthenia gravis, acute infectious polyneuritis, or poliomyelitis.
- Central nervous system conditions, such as head trauma, anesthetic toxicity, improper use of mechanical ventilation, or excessive inhalation of CO2.
In respiratory acidosis, renal compensation increases blood bicarbonate levels. Simultaneously, the kidneys secrete acidic urine and increase chloride excretion, leading to lowered blood chloride levels. When PaCO2 is below 60 mmHg, compensation may keep pH within the normal range. Respiratory acidosis is often accompanied by hypoxemia and respiratory distress. Severe hypercapnia can cause vasodilation and increased cerebral blood flow, resulting in headache and elevated intracranial pressure. Profound hypercapnia may lead to central nervous system suppression and decreased blood pH.
Clinical Manifestations
Symptoms include those of the underlying disease along with hypoxemia and respiratory distress. Hypercapnia leads to vasodilation and increased cerebral blood flow, causing headache and elevated intracranial pressure. Severe cases may present with central nervous system suppression.
Management Principles
Treatment primarily involves addressing the underlying disease. Artificial ventilation may be required if necessary.
Respiratory Alkalosis
Respiratory alkalosis is characterized by excessive alveolar ventilation, resulting in a significant reduction of CO2 and a concomitant decrease in H2CO3 levels. Major causes include:
- Hyperventilation caused by factors such as anxiety, prolonged crying, rapid breathing due to fever, psychological conditions, or improper use of mechanical ventilation resulting in excessive CO2 elimination.
- Neurological conditions, such as meningitis, brain tumors, or trauma.
- Early-stage salicylate poisoning.
- Carbon monoxide poisoning.
Clinical Manifestations
Symptoms primarily include deep and rapid breathing and are otherwise similar to those seen in metabolic alkalosis. Blood gas analysis reveals increased pH, decreased PaCO2, reduced HCO3- concentration, and acidic urine.
Management Principles
Respiratory alkalosis is managed by treating the underlying cause.
Mixed Acid-Base Disorders
Mixed acid-base disorders occur when two or more types of acid-base imbalances coexist. Respiratory acidosis combined with metabolic acidosis is a common type of mixed acid-base imbalance. Examples include diabetic ketoacidosis coexisting with emphysema and respiratory distress syndrome (RDS) accompanied by both respiratory and metabolic acidosis. In such cases, there may be concurrent reductions in HCO3- levels and CO2 retention, leading to a marked decrease in blood pH. Chronic respiratory acidosis accompanied by congestive heart failure and excessive use of diuretics can result in metabolic alkalosis, causing plasma HCO3- levels and pH to rise above those typically seen in isolated chronic respiratory acidosis. In cases of hepatic failure, metabolic acidosis may coexist with respiratory alkalosis, where pH changes may be minimal, despite significant decreases in plasma HCO3- levels and PaCO2.
Management of Mixed Acid-Base Disorders
Treatment is to address the underlying primary diseases, maintain airway patency, and use artificial ventilation when required to normalize pH.
Management of high anion gap metabolic acidosis focuses on correcting hypoxia, controlling infection, and improving circulation. Metabolic acidosis may resolve or improve after mechanical ventilation enhances lung function; only a small number of patients require alkalizing agents. When prescribing alkalizing agents, adequate ventilation must be ensured. In cases of significantly low pH, immediate use of alkalizing agents is needed.
Clinical Assessment of Acid-Base Balance
The clinical assessment of acid-base balance is performed using three indicators: blood pH, PaCO2, and HCO3- concentration. Both pH and PaCO2 can be measured directly, and while HCO3- can also be measured directly, it is often estimated using the total serum CO2 content. It should be noted that common blood gas analyzers generally measure only pH, PaCO2, and PaO2 with specific electrodes, while HCO3- is calculated using the Henderson-Hasselbalch equation.
Determining simple acid-base imbalances is relatively straightforward, as pH changes depend on the ratio of PaCO2 to HCO3-. Clinically, the evaluation begins by identifying whether acidosis or alkalosis is present. Next, it is determined whether the imbalance is respiratory or metabolic in origin. For metabolic acidosis, it is further analyzed to determine whether it is a high-anion gap or normal-anion gap type. Finally, the adequacy of respiratory or metabolic compensation is assessed.
Commonly Used Fluid Solutions in Fluid Therapy
Commonly used fluids include non-electrolyte and electrolyte solutions. Non-electrolyte solutions often involve 5% or 10% glucose solutions, which are considered hypotonic solutions because glucose is metabolized into water after entering the body. Electrolyte solutions include sodium chloride, potassium chloride, sodium lactate, sodium bicarbonate, ammonium chloride, and their various preparations.
Oral rehydration salts (ORS) were recommended by the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) starting in 1971 for the treatment of acute diarrhea accompanied by dehydration. The theoretical basis involves the sodium-glucose coupled transport absorption mechanism of the small intestine. Sodium-glucose cotransporters located on the brush border of small intestinal epithelial cells facilitate the simultaneous binding of sodium and glucose, substantially increasing the absorption of sodium and water.
Given that most cases of dehydration caused by diarrhea in infants and young children are isotonic, WHO has recommended the use of a low-osmolarity ORS formula since 2002. The formula includes Na+ 75 mmol/L, K+ 20 mmol/L, Cl- 65 mmol/L, citrate 10 mmol/L, and glucose 75 mmol/L, and is prepared by dissolving 2.6 g of sodium chloride, 2.9 g of sodium citrate, 1.5 g of potassium chloride, and 13.5 g of glucose in 1,000 mL of water, yielding a total osmolarity of 245 mOsm/L.
ORS is suitable for patients with mild to moderate dehydration without severe vomiting. The typical dosage is 50 mL/kg for mild dehydration and 100 mL/kg for moderate dehydration, administered over 4 hours. The amount required for ongoing losses depends on the further fluid loss caused by diarrhea, with an additional 10 mL/kg given after each episode of diarrhea. ORS should not be used in cases of extreme lethargy, coma, somnolence, or abdominal distension. For ongoing losses and physiological requirements, ORS may need to be appropriately diluted.
Fluid Therapy
The goal of fluid therapy is to maintain or restore normal fluid volume and composition to ensure proper physiological function. Fluid therapy involves replacing physiological requirements, accumulated deficits, and ongoing losses. Each component can be calculated and replaced independently. For example, children undergoing surgery while fasting may only need to replace their physiological requirements and electrolytes, whereas patients with diarrhea require replacement of physiological requirements, accumulated deficits, and ongoing losses.
In creating a fluid therapy plan, a comprehensive understanding of the patient’s medical history, physical examination, laboratory results, and individual differences is required. Consideration of the body's compensatory mechanisms is essential, and patients with organ dysfunction, including renal, pulmonary, or cardiac impairment, should receive carefully selected fluids with attention to the volume and infusion rate. Regular monitoring and timely adjustments to treatment are necessary based on changes in patient condition.
Replacement of Physiological Requirements
Physiological requirements include water, electrolytes, and caloric needs. Water requirements encompass sensible water loss (via urine and stool) and insensible water loss (via skin and lungs). Insensible water loss accounts for 35%, urine output for 60%, and stool for 5% of daily water requirements. Daily water needs are generally calculated based on calorie expenditure, with 100–150 mL of water required for every 100 kcal of metabolic energy. Younger age groups require relatively larger amounts of water, so water needs can also be calculated based on body weight. In children with fever or rapid breathing, fluid intake may need to be increased, as insensible water loss rises by 12% for every degree Celsius over 38°C. Rapid breathing and tracheostomy can also increase pulmonary insensible water loss. Extremely low birth weight infants may require over 100 mL/kg per day of insensible water loss replacement.
Electrolyte requirements include replenishing electrolytes lost through normal sweating, bowel movements, and physiological consumption. The needs vary significantly, but the average daily consumption of potassium, sodium, and chloride is about 2–3 mmol per 100 kcal.
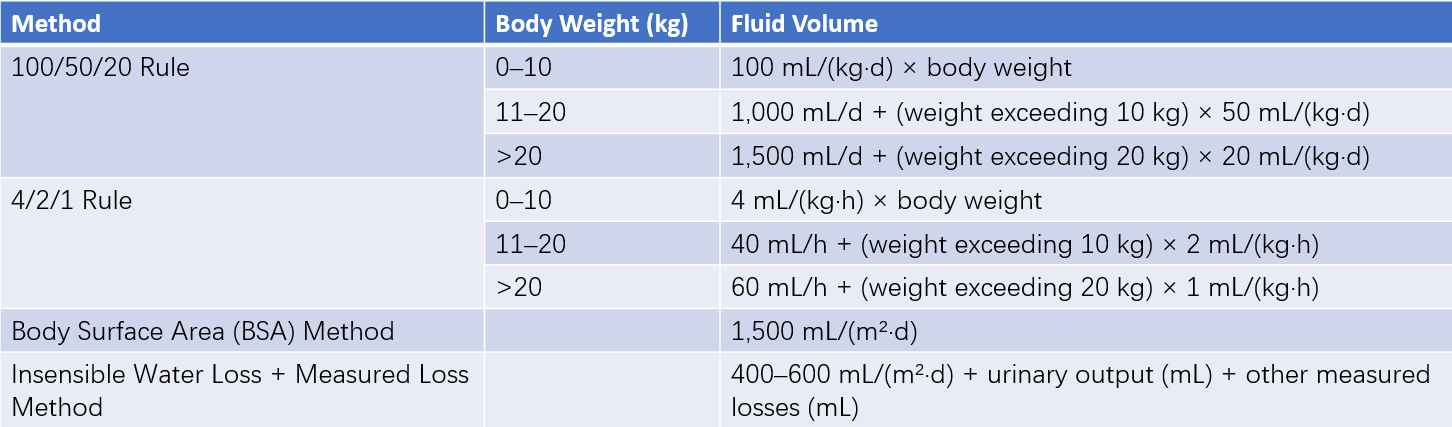
Table 6 Four methods for calculating physiological fluid requirements
Whenever possible, the physiological requirements should be replenished orally. For cases where oral intake is insufficient or not possible, intravenous infusion of 1/4 to 1/5 isotonic sodium-containing solution supplemented with the required potassium is an option. Nutritional deficiencies should be addressed by providing adequate calories and protein, with partial or total parenteral nutrition utilized when necessary.
Replacement of Accumulated Deficits
Accumulated deficits refer to the total volume of fluids lost since the onset of illness. Replenishment is adjusted based on the degree and type of dehydration. Fluid replacement for accumulated deficits is as follows:
- Mild dehydration: 30–50 mL/kg of body weight.
- Moderate dehydration: 50–100 mL/kg.
- Severe dehydration: 100–120 mL/kg.
For hypotonic dehydration, 2/3 isotonic sodium-containing solutions are used; for isotonic dehydration, 1/2 isotonic sodium-containing solutions are applied; and for hypertonic dehydration, 1/3 to 1/5 isotonic sodium-containing solutions are appropriate. When the type of dehydration is uncertain, isotonic dehydration treatment is often initiated.
The infusion rate depends on the severity of dehydration, and the standard approach involves starting with a rapid infusion and then slowing down. For children with severe dehydration accompanied by poor circulation and shock, isotonic sodium-containing solutions such as normal saline or 2:1 isotonic solutions are infused at 20 mL/kg over 0.5–1 hour. The remaining accumulated deficits are typically replaced within 8–12 hours. Potassium supplementation is initiated once circulation improves and diuresis begins. Correction of acid-base imbalances and other electrolyte abnormalities follows guidelines outlined under acid-base balance disorders.
For hypertonic dehydration, the correction of hypernatremia must proceed slowly, with serum sodium levels decreasing by less than 10 mmol/L every 24 hours. The correction may take several days, and higher or even isotonic tension fluids may be required at times to prevent cerebral edema resulting from rapid sodium reduction.
Replacement of Ongoing Losses
After addressing accumulated deficits, ongoing losses caused by diarrhea, vomiting, gastrointestinal drainage, and other factors often persist, resulting in continued fluid depletion. Replacement of ongoing losses varies depending on the underlying cause, following the principle of replacing exactly what is lost with an equivalent amount.
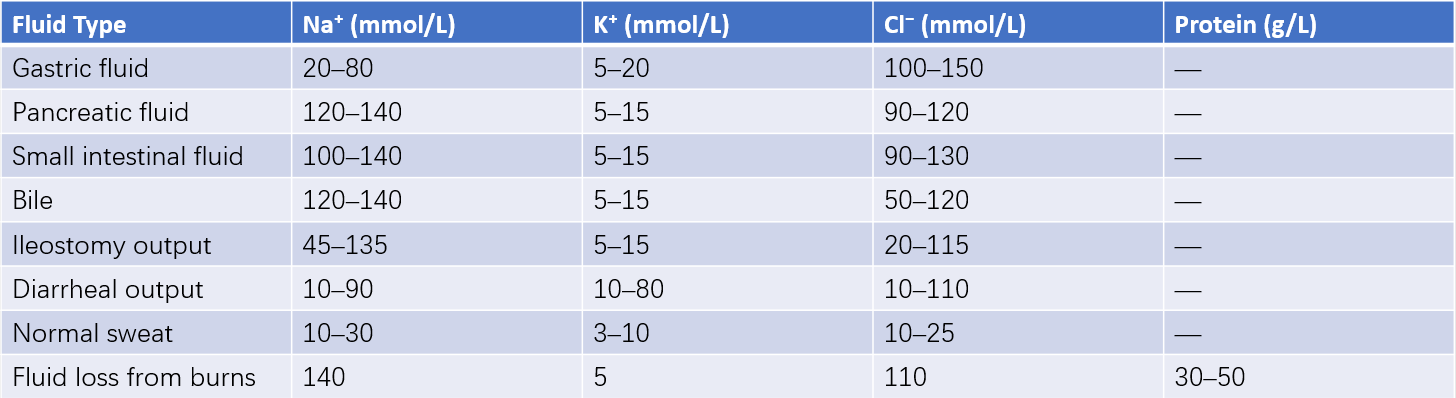
Table 7 Composition of fluid loss in various body fluids