Hormones in the female reproductive endocrine system include those secreted by the hypothalamus, pituitary gland, and ovaries. The hormones secreted by these organs regulate and influence each other to maintain normal physiological functions. For instance, gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus regulates ovarian function by modulating the secretion of gonadotropins from the pituitary gland. Conversely, sex hormones secreted by the ovaries exert feedback regulation on the hypothalamic-pituitary axis. Therefore, measurement of hormone levels within the hypothalamic-pituitary-ovarian axis holds significant value for the diagnosis of certain diseases, evaluation of therapy, prognosis assessment, and in the study of reproductive physiology and the development of contraceptive drugs.
Insulin secreted by the pancreas not only participates in glucose metabolism but also plays an important role in maintaining normal ovarian function. Insulin resistance contributes significantly to the development of conditions such as polycystic ovary syndrome (PCOS), endometrial cancer, and gestational diabetes mellitus. Elevated insulin levels in the body can stimulate excessive androgen production by the ovaries, resulting in hyperandrogenemia, menstrual irregularities, or even amenorrhea. Tests such as oral glucose tolerance test (OGTT) and insulin release test may serve as auxiliary diagnostic and therapeutic tools for these conditions.
Hormone levels are typically measured from peripheral venous blood using methods such as gas chromatography, spectrophotometry, fluorescence detection, enzyme-linked immunosorbent assay (ELISA), and radioimmunoassay. In recent years, non-radioactive immunochemiluminescence methods have also gained wider application.
Measurement of Hypothalamic Gonadotropin-Releasing Hormone (GnRH)
Gonadotropin-releasing hormone (GnRH), a decapeptide hormone secreted by neural cells in the arcuate nucleus of the hypothalamus, is transported directly to the anterior pituitary through the pituitary portal system, where it regulates the synthesis and secretion of gonadotropins. The synthetic decapeptide GnRH stimulates the secretion of luteinizing hormone (LH) more strongly than follicle-stimulating hormone (FSH); hence, it is also referred to as luteinizing hormone-releasing hormone (LHRH).
The most notable hormonal fluctuation during a normal female menstrual cycle is the mid-cycle pre-ovulatory LH surge. However, the low concentration and short half-life of GnRH in peripheral blood make its direct measurement challenging. Instead, GnRH function is assessed using the GnRH stimulation test (also known as the pituitary stimulation test) or the clomiphene challenge test to evaluate hypothalamic and pituitary function as well as the associated pathophysiological states.
GnRH Stimulation Test
Principle
LHRH promotes the release of gonadotropins from the pituitary gland. After an exogenous LHRH injection, gonadotropin levels in peripheral blood are measured at different time points to assess pituitary function. A normal pituitary gland exhibits a reactive increase in gonadotropin levels, whereas impaired pituitary function results in a weak or delayed reaction, with little to no increase in gonadotropin levels.
Method
LHRH (100 μg) dissolved in 5 ml of 0.9% sodium chloride solution is injected intravenously at 8:00 AM. Peripheral venous blood samples (2 ml each) are collected at baseline (before injection) and at 30, 60, and 90 minutes post-injection to measure FSH and LH levels.
Result Analysis
Normal Response
LH increases 2–3 times above baseline, with a peak occurring within 15–30 minutes.
Hyperactive Response
Peak LH levels increase more than fivefold compared to baseline.
Delayed Response
The time to peak LH levels is longer than that observed in a normal response.
No or Weak Response
LH levels show little or no change post-injection, with an increase of less than twofold compared to baseline.
Clinical Significance
Delayed Puberty
Normal response is observed in GnRH stimulation tests.
Pituitary Insufficiency
Conditions such as Sheehan's syndrome, pituitary tumors, or empty sella syndrome, which disrupt pituitary tissue, manifest as no or weak response in GnRH stimulation tests.
Hypothalamic Dysfunction
Delayed or normal responses may occur, commonly seen in hypothalamic amenorrhea.
Ovarian Insufficiency
Baseline FSH and LH levels exceed 30 IU/L, and a hyperactive response is observed in GnRH stimulation tests.
Polycystic Ovary Syndrome (PCOS)
LH/FSH ratio is 2–3 or higher, with a hyperactive response in GnRH stimulation tests.
Clomiphene Challenge Test
Principle
Clomiphene, also known as clomifene, is a non-steroidal estrogen antagonist with weak estrogenic effects. It binds to estrogen and androgen receptors in the hypothalamus, blocking the negative feedback action of estrogen on the hypothalamus and/or the anterior pituitary gland, thereby stimulating the hypothalamus to release gonadotropin-releasing hormone (GnRH). The clomiphene challenge test is used to evaluate the function of the hypothalamic-pituitary-ovarian (HPO) axis in patients with amenorrhea and to differentiate between hypothalamic and pituitary disorders.
Method
Starting on the fifth day of the menstrual cycle, clomiphene is administered orally at a dose of 50–100 mg per day for five consecutive days. After administration, luteinizing hormone (LH) may increase by approximately 85%, and follicle-stimulating hormone (FSH) may increase by about 50%. Following discontinuation, LH and FSH levels decrease. If a subsequent rise in LH to pre-ovulatory levels occurs, it indicates an ovulatory response, with ovulation typically occurring between the fifth and ninth day after medication discontinuation. If no subsequent rise in LH levels is observed within 20 days after discontinuation, it indicates a lack of response. Blood levels of LH and FSH are measured on the 1st, 3rd, and 5th days of medication administration, with progesterone levels assessed in the third week or during the premenstrual phase.
Clinical Significance
Hypothalamic Disorders
Non-responsiveness to the clomiphene test combined with responsiveness to the GnRH stimulation test indicates hypothalamic dysfunction.
Delayed Puberty
GnRH stimulation tests can be used to determine whether delayed puberty is caused by hypothalamic or pituitary dysfunction.
Measurement of Pituitary Gonadotropins
Source and Physiological Function
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are glycoprotein hormones secreted by gonadotroph cells in the anterior pituitary gland. These hormones circulate in the blood bound to α2 and β globulins and are regulated by hypothalamic GnRH, ovarian hormones, and inhibins. In women of reproductive age, pituitary gonadotropins exhibit cyclic fluctuations in accordance with the menstrual cycle. The primary function of FSH involves promoting follicular maturation and estrogen secretion, while LH plays a key role in inducing ovulation and corpus luteum formation, which facilitates the production of progesterone and estrogen by the corpus luteum.
Normal Values
These can be seen in Tables 1 and 2 for reference ranges.
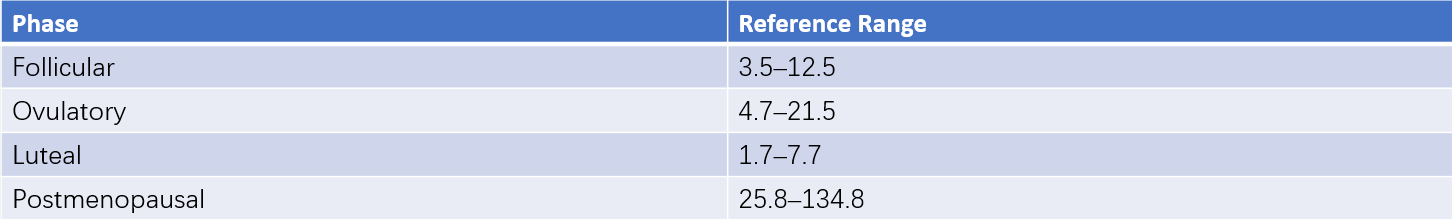
Table 1 Reference ranges for serum FSH (ECLIA method) (Unit: IU/L)
Note: ECLIA (electrochemiluminescence immunoassay) refers to the electrochemical luminescence immunoassay.
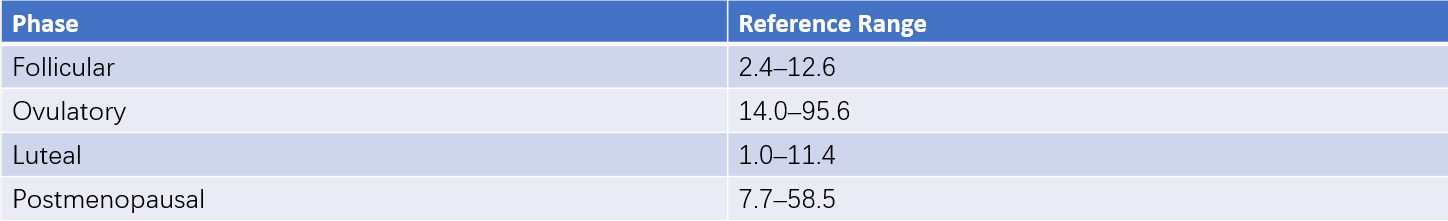
Table 2 Reference ranges for serum LH (ECLIA method) (Unit: IU/L)
Clinical Applications
Differentiation of Amenorrhea Causes
Decreased levels of both FSH and LH suggest a primary cause in the anterior pituitary or hypothalamus, while elevated FSH and LH levels indicate ovarian dysfunction.
Ovulation Monitoring
Assessment of the LH peak is useful for estimating ovulation timing and evaluating ovulatory function, aiding in the diagnosis of infertility and research on contraceptive mechanisms.
Diagnosis of Polycystic Ovary Syndrome (PCOS)
An LH/FSH ratio of ≥2–3 provides diagnostic support for PCOS.
Diagnosis of Precocious Puberty
Useful for distinguishing central precocious puberty from pseudo-precocious puberty. Central precocious puberty involves increased gonadotropin secretion with cyclical changes in FSH and LH, while pseudo-precocious puberty is characterized by low, non-cyclic FSH and LH levels.
Premature Ovarian Failure
FSH levels >40 IU/L measured at least twice within a one-month interval confirm the diagnosis.
Measurement of Pituitary Prolactin
Source and Physiological Function
Prolactin (PRL) is a polypeptide hormone secreted by lactotroph cells in the anterior pituitary gland. It is regulated by hypothalamic prolactin-inhibiting hormones (primarily dopamine) and prolactin-releasing factors. Additional modulatory factors, such as thyrotropin-releasing hormone (TRH), estrogen, and serotonin, also promote its release. In peripheral blood, PRL exists in four molecular isoforms: monomeric PRL (small PRL), big PRL, macro-PRL, and variant PRL. Only monomeric PRL, which accounts for 80% of total secreted PRL, possesses biological activity. Laboratory measurements encompass the total levels of all PRL isoforms, which do not always correlate with biological activity. For example, hyperprolactinemia may occur without milk secretion, while normoprolactinemia may coexist with lactation.
The main functions of PRL include promoting mammary gland development and lactation, as well as collaborating with ovarian steroid hormones to facilitate the development of mammary ducts and alveoli during late pregnancy. PRL also plays regulatory roles in various physiological processes, particularly reproductive function.
Normal Values
The normal range of blood PRL varies based on physiological states:
- Non-pregnant: <25 μg/L (530 mIU/L).
- Early pregnancy: <80 μg/L (1,696 mIU/L).
- Mid-pregnancy: <160 μg/L (3,392 mIU/L).
- Late pregnancy: <400 μg/L (8,480 mIU/L).
Clinical Applications
Measurement of PRL levels is critical for evaluating amenorrhea, infertility, and menstrual irregularities, regardless of the presence or absence of galactorrhea, to rule out hyperprolactinemia.
Abnormal elevations of PRL in patients with pituitary tumors may indicate a prolactinoma.
PRL elevations are also observed in conditions such as precocious puberty, primary hypothyroidism, prolonged lactation, neuropsychiatric stimuli, and medication use (e.g., chlorpromazine, contraceptives, high-dose estrogen, reserpine). Decreased PRL levels are often associated with pituitary hypofunction or isolated prolactin-secretion deficiencies.
Approximately 20%–25% of PCOS patients exhibit mild hyperprolactinemia, possibly due to sustained estrogen stimulation.
Measurement of Estrogens
Source and Physiological Changes
In women of reproductive age, estrogens are primarily produced by the ovaries, while in pregnant women, estrogens originate mainly from the ovaries and placenta, with small amounts being secreted by the adrenal glands. Estrogens consist of estrone (E1), estradiol (E2), and estriol (E3). Among them, estradiol (E2) is the most potent and acts as one of the primary sex hormones secreted by the ovaries, playing an essential role in maintaining female reproductive function and secondary sexual characteristics. Estriol (E3) is a metabolic product of estrone and estradiol. During pregnancy, the placenta produces large amounts of estriol, and measuring E3 levels in blood or urine can reflect placental and fetal function. Estrogens are metabolized and inactivated in the liver and excreted by the kidneys.
In prepubertal girls, estrogen levels are relatively low. In sexually mature women, estradiol levels fluctuate in accordance with the ovarian cycle in a normal menstrual period. Estrogen levels are at their lowest during the early follicular phase but gradually rise, reaching a peak just before ovulation, followed by a decline. Another rise occurs roughly 7–8 days after ovulation (second peak), which is lower than the pre-ovulatory peak. Thereafter, estrogen levels rapidly drop to their baseline. In postmenopausal women, ovarian function declines, resulting in estradiol levels below early follicular phase values. In this phase, most estrogens are derived from the peripheral conversion of androstenedione.
Normal Values
These can be seen in Table 3 for reference values.
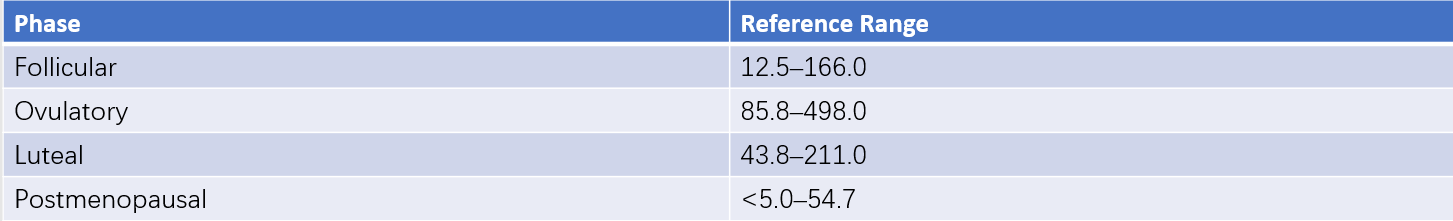
Table 3 Reference ranges for serum estradiol (E2) (ECLIA method) (Unit: ng/L)
Clinical Applications
Monitoring Ovarian Function
Blood levels of estradiol (E2) or 24-hour urinary total estrogens can be measured.
Assessment of Amenorrhea Causes
Normal cyclical variations in estrogen levels suggest normal follicular development, pointing to uterine causes of amenorrhea. Low estrogen levels may indicate primary or secondary ovarian insufficiency or ovarian suppression due to medication.
Monitoring Follicular Development
Estradiol levels in blood serve as indicators of follicular growth and maturation during ovulation induction therapy and can guide the timing of human chorionic gonadotropin (hCG) administration and egg retrieval.
Determination of Ovulation
Absence of ovulation is accompanied by the lack of cyclic changes in estrogen levels, commonly observed in anovulatory abnormal uterine bleeding, polycystic ovary syndrome (PCOS), or certain postmenopausal cases of uterine bleeding.
Diagnosis of Female Precocious Puberty
Clinical manifestations of secondary sexual characteristics before the age of 7.5 years, along with elevated estradiol levels, indicate precocious puberty.
Assisting in PCOS Diagnosis
Elevated estrone (E1), normal or slightly elevated estradiol (E2) levels, consistent with early follicular phase values, and an E1/E2 ratio >1 are suggestive of PCOS.
Monitoring Fetal-Placental Unit Function
During pregnancy, estriol (E3) is predominantly produced by the fetal-placental unit. Measuring urinary E3 levels in pregnant women reflects placental and fetal function. Normal E3 excretion increases rapidly from the 29th week of gestation, with an average excretion of 88.7 nmol/24 h during full-term pregnancies. Persistent urinary E3 excretion rates <37 nmol/24 h or a sudden decrease of >30%–40% after 36 weeks may indicate placental deterioration. Levels <22.2 nmol/24 h or a sudden decrease of >50% suggest significant placental dysfunction.
Measurement of Progesterone
Source and Physiological Function
In females, progesterone is produced by the ovaries, placenta, and adrenal cortex. Its levels fluctuate with the menstrual cycle. During the follicular phase, progesterone levels remain very low. After ovulation, the corpus luteum in the ovary produces large amounts of progesterone, causing levels to rise rapidly, peaking 6–8 days after the LH surge. Levels then decline to follicular phase values about four days before menstruation. During pregnancy, serum progesterone levels steadily increase as the pregnancy progresses; in the first six weeks, progesterone is mainly produced by the corpus luteum, while in the mid-to-late stages of pregnancy, the placenta becomes the main source. Progesterone acts primarily in conjunction with estrogen to transform the endometrium into the secretory phase, enabling its periodic shedding to form menstruation. During pregnancy, it facilitates embryo implantation, prevents uterine contraction, and helps maintain uterine quiescence until labor. Additionally, progesterone promotes the development of mammary alveoli to prepare for lactation.
Normal Values
These can be seen in Table 4 for reference values.
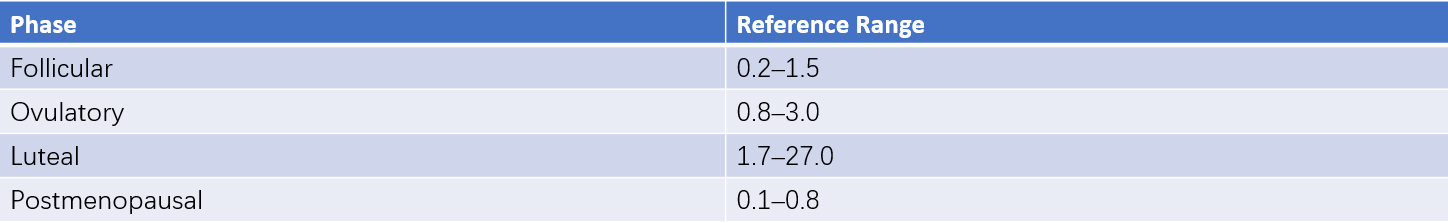
Table 4 Reference ranges for serum progesterone (ECLIA method) (Unit: μg/L)
Clinical Applications
Ovulation Monitoring
Blood progesterone levels >5 μg/L indicate ovulation. Progesterone levels can be used to evaluate the efficacy of ovulation induction medications. If progesterone levels indicate ovulation but infertility persists for other reasons, ultrasound monitoring of follicular development and ovulation may help exclude luteinized unruptured follicle syndrome (LUFS). Other contributing factors, such as primary or secondary amenorrhea, anovulatory menstrual cycles, anovulatory uterine bleeding, PCOS, oral contraceptives, or prolonged use of GnRH agonists, may result in reduced progesterone levels.
Assessment of Luteal Function
Progesterone levels below physiological values during the luteal phase suggest luteal insufficiency. If levels remain elevated four to five days before menstruation, incomplete luteal regression may be indicated.
Supporting Diagnosis of Threatened Miscarriage
Low progesterone levels within the first 12 weeks of pregnancy are associated with a higher risk of spontaneous abortion. Decreasing trends in progesterone levels during a threatened miscarriage suggest a greater likelihood of miscarriage.
Evaluation of Placental Function
Reduced progesterone levels in pregnancy may reflect impaired placental function. A single serum progesterone level ≤5 μg/L (15.9 nmol/L) indicates a risk of fetal death.
Monitoring Progesterone Replacement Therapy
Serum progesterone levels should be monitored during natural progesterone replacement therapy.
Measurement of Androgens
Source and Physiological Changes
Androgens in females are secreted by the ovaries and adrenal cortex. Androgens primarily include testosterone and androstenedione. Testosterone is mainly produced through the conversion of androstenedione secreted by the ovaries and adrenal glands. Approximately 50% of androstenedione originates from the ovaries, while the other 50% is derived from the adrenal cortex. Its biological activity lies between that of testosterone, which is highly active, and dehydroepiandrosterone (DHEA), which has weak activity. Serum DHEA is primarily secreted by the adrenal cortex. Before menopause, serum testosterone serves as a marker of ovarian androgen production, whereas after menopause, the adrenal cortex becomes the primary source of androgen production.
Normal Values
Using the electrochemiluminescence immunoassay (ECLIA) method, the normal reference range varies by age group:
- Ages 20–49: 0.084–0.481 μg/L.
- Ages ≥50: 0.029–0.408 μg/L.
Clinical Applications
Ovarian Virilizing Tumors
The sudden onset and progressive worsening of androgen excess symptoms in women, along with elevated serum androgen levels, often indicate the presence of ovarian virilizing tumors.
Polycystic Ovary Syndrome (PCOS)
Testosterone levels typically do not exceed twice the upper limit of the normal range, while androstenedione is often elevated, and DHEA is normal or slightly elevated. Elevated androgen levels prior to treatment are expected to decline after treatment, making serum androgen levels a useful marker for evaluating treatment effectiveness.
Adrenal Cortex Hyperplasia or Tumors
Serum androgen levels are abnormally elevated.
Disorders of Sexual Development
Male pseudohermaphroditism and true hermaphroditism are characterized by testosterone levels within the normal range for males.
Female pseudohermaphroditism presents with testosterone levels within the normal range for females.
Use of Androgen or Androgenic Drugs
Monitoring androgen levels may be necessary during the use of androgen preparations or endocrine medications with androgenic properties, such as danazol.
Hirsutism in Women
Normal serum testosterone levels in women with hirsutism often suggest increased sensitivity of hair follicles to androgens.
Measurement of Human Chorionic Gonadotropin (hCG)
Source and Physiological Changes
Human chorionic gonadotropin (hCG) is a glycoprotein hormone composed of α and β subunits. It is primarily produced by trophoblastic cells during pregnancy and is often elevated in cases of pregnancy, gestational trophoblastic disease, germ cell tumors, and other malignant neoplasms. Recent observations suggest that serum hCG fluctuations parallel the pulsatile secretion of luteinizing hormone (LH) and may also increase during the mid-menstrual cycle, indicating that hCG is partially secreted by the pituitary gland. These factors should be considered in clinical analyses.
In normal pregnancy, hCG production begins as early as the 6th day after ovulation when the trophoblast forms. Detectable levels of hCG in peripheral blood are observed approximately one day later. hCG levels double approximately every 1.7 to 2 days, reaching about 100 IU/L by 14 days post-ovulation. Peak levels of 50,000–100,000 IU/L are typically reached between 8 and 10 weeks of gestation, after which levels decline rapidly, falling to approximately 10% of the peak value during mid-to-late pregnancy. Due to the structural similarity between the α-subunits of hCG and LH, β-hCG is sometimes measured to avoid cross-reactivity.
Normal Values
These can be seen in Table 5 for details.
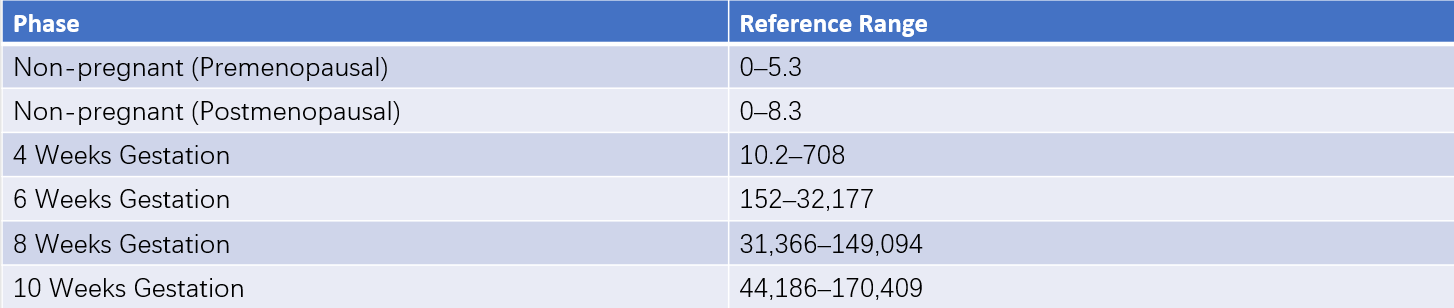
Table 5 Reference ranges for serum hCG concentration (ECLIA method) (Unit: IU/L)
Clinical Applications
Pregnancy Diagnosis
Quantitative immunoassays for serum hCG are used for early pregnancy detection due to their rapidity, simplicity, and cost-effectiveness. Early pregnancy test strips, widely used for convenience, can detect urinary hCG as low as 25 IU/L.
Ectopic Pregnancy
Persistent low serum hCG levels that fail to rise significantly over 2–3 days may indicate ectopic pregnancy.
Gestational Trophoblastic Disease
Serum hCG measurement supports the diagnosis, treatment, and follow-up monitoring of trophoblastic diseases.
Tumors Secreting hCG
Germinomas of the hypothalamus or pineal gland, ovarian nonspecific germ cell tumors, immature teratomas, and other tumors—such as colorectal, liver, lung, and stomach cancers—may secrete hCG. These tumors can lead to menstrual irregularities in adult women. Sudden onset of menstrual disturbances accompanied by elevated hCG levels warrants consideration of ectopic tumor secretion.
Oral Glucose Tolerance Test (OGTT) — Insulin Release Test
Principle
Insulin secretion occurs in two forms: basal secretion during fasting states and stimulated secretion in response to various stimuli. Glucose is the most potent stimulator of insulin secretion. Simultaneous measurement of plasma insulin during OGTT provides information about pancreatic β-cell function and potential insulin resistance.
Method
After 8–12 hours of fasting, fasting venous blood is collected to measure plasma glucose and insulin levels. Venous blood is subsequently drawn at 30, 60, 120, and 180 minutes after the oral administration of 75 g of glucose to measure plasma glucose and insulin levels.
Test Results and Analysis
These can be seen in Table 6 for results.
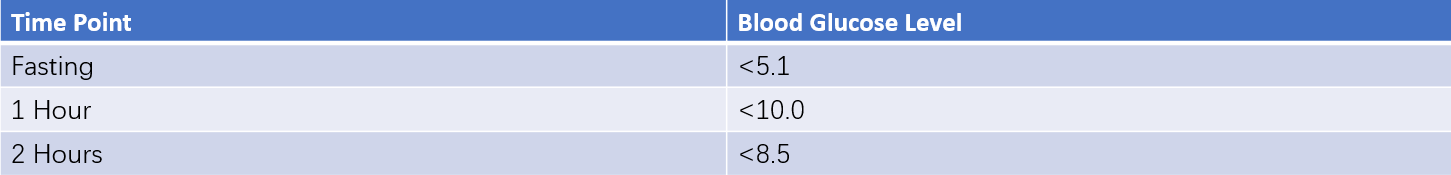
75g oral glucose tolerance test (OGTT) blood glucose levels (Unit: mmol/L)
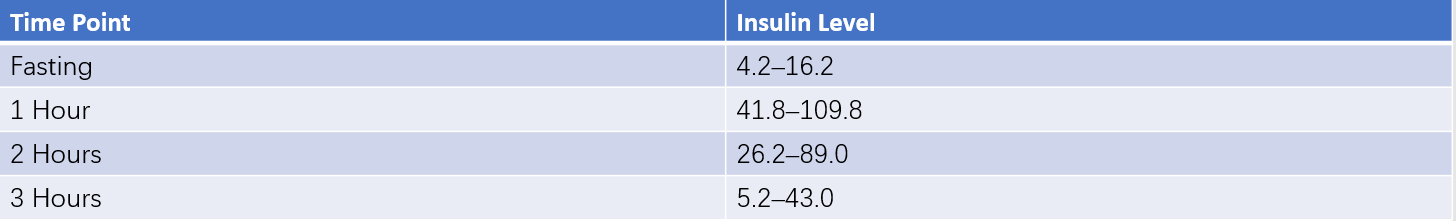
Insulin release test (75g oral glucose load) (Unit: mU/L)
Table 6 Reference ranges for OGTT and insulin release test
Analysis of the results is as follows:
- Normal Response: Basal plasma insulin in healthy individuals typically ranges from 5–20 mU/L. Insulin levels peak at 30–60 minutes after glucose administration (5–10 times the basal level, often 50–100 mU/L) and gradually decline, returning to basal levels within 3 hours.
- Insulin Insufficiency: Both fasting insulin levels and glucose-stimulated insulin secretion are absolutely insufficient, indicating significant pancreatic β-cell dysfunction or damage.
- Insulin Resistance: Fasting glucose and insulin levels are elevated, and both glucose and insulin levels post-glucose administration are significantly higher than normal, indicating insulin resistance.
- Delayed Insulin Secretion: Normal or elevated fasting insulin levels with delayed peak insulin levels following glucose administration are characteristic of type 2 diabetes.
Clinical Significance
Diabetes Classification
The insulin release test, combined with clinical history and symptoms, aids in the classification of diabetes. Insufficient insulin secretion suggests severe pancreatic damage and may indicate type 1 diabetes, while delayed insulin secretion is a hallmark of type 2 diabetes.
Supporting Diagnosis of Certain Gynecological Disorders
Hyperinsulinemia and insulin resistance are useful in diagnosing conditions such as PCOS and endometrial cancer.