Reproductive tract exfoliated cells in females primarily refer to epithelial cells from the vagina, cervix, uterus, and fallopian tubes. Clinically, the examination of exfoliated epithelial cells from the reproductive tract serves as a means to assess physiological and pathological changes. These exfoliated epithelial cells mainly originate from the upper vagina and the ectocervix, with contributions from the uterus, fallopian tubes, and epithelial cells from the pelvic-abdominal cavity. This method aids in diagnosing malignancies at different locations within the reproductive tract and monitoring therapeutic outcomes. It is a simple, economical, and practical auxiliary diagnostic tool. However, the presence of malignant cells detected through reproductive tract cytology only suggests a preliminary screening and does not provide precise localization; further examinations are required for definitive diagnosis. Conversely, the absence of malignant cells does not completely rule out the possibility of malignancy and necessitates a comprehensive consideration of other diagnostic results. Historically, reproductive tract cytology has also been used in the auxiliary diagnosis of gynecological endocrine disorders and miscarriages, although its current application in these areas has decreased.
Sample Collection, Slide Preparation, and Related Techniques in Reproductive Tract Cytology
Types of Smears and Sample Collection
Pre-Sampling Preparation
Intercourse, vaginal douching, and the use of any vaginal medications should not occur within 24 hours before sampling. In cases of acute lower genital tract infections, sampling is recommended only after the infection has been treated. Prior to sample collection, the use of vaginal lubricants, acetic acid, or iodine solutions should be avoided.
Cervical Smears
This technique is used for obtaining exfoliated cells from the cervix. Samples are collected from the squamous-columnar junction at the ectocervix and inside the cervical canal, primarily for cervical cancer screening. Earlier methods employed wooden spatulas, which were less effective in collecting sufficient cell samples and often resulted in suboptimal smear quality. Currently, cervical brushes with longer central bristles flanked by shorter peripheral bristles are widely used. After cleaning cervical surface secretions, the brush is inserted so that the longer bristles reach inside the cervical canal and the shorter bristles contact the ectocervix, covering the entire transformation zone. Gentle rotation in a single direction for at least five full turns is performed before removing the brush. The collected sample is immediately transferred into a preservation solution, placed with the brush head in a container with the solution, or spread onto a slide. Smear preparation methods include conventional smears and liquid-based cytology (LBC). Compared to conventional methods, LBC improves sample collection, eliminates interference from blood, and evenly distributes cells on the slide. The single-layered cell smears produced by LBC offer clearer cellular and background features, more stable quality, and easier cytological interpretation. Additionally, this technology supports automated slide reading, enables multiple preparations with a single collection, and allows for HPV testing using compatible preservation solutions.
Intrauterine Cytological Sampling
When malignancy within the uterine cavity is suspected, sampling can be performed using an endometrial sampling device (vacuum or brush-based). The device is introduced into the uterine cavity up to the fundus, and cells are gently brushed or aspirated by rotating the device in multiple directions. The collected samples are then prepared as smears, fixed, and stained. This simple method provides effective samples and is suitable for women experiencing abnormal uterine bleeding. Compared to diagnostic curettage, it is less painful and more acceptable to patients, although its sample coverage may be incomplete. It is important to note that samples from the uterine cavity may include epithelial components from the fallopian tubes, ovaries, or pelvic-abdominal cavity.
Staining Methods
Various staining methods are available for cytological evaluation, including the Papanicolaou stain (Pap stain), Shao stain, and other modified staining techniques. The Pap stain is most commonly utilized.
Auxiliary Diagnostic Techniques
Auxiliary diagnostic methods include immunocytochemistry, in situ hybridization, imaging analysis, flow cytometry, methylation assays, next-generation sequencing (NGS), as well as automated screening and artificial intelligence-assisted diagnostic systems.
Morphological Characteristics of Normal Reproductive Tract Exfoliated Cells
Squamous Epithelial Cells
The squamous epithelium of the vagina and ectocervix are of similar composition, consisting of non-keratinized stratified squamous epithelium. The epithelial cells are organized into basal, intermediate, and superficial layers. Their growth and maturation are influenced by estrogen secreted from the ovaries, and the proportion of each layer varies across different life stages and phases of the menstrual cycle. Cells mature from the basal layer toward the superficial layer, with the following progressive changes: cell size increases, cell shape evolves from round to boat-shaped or polygonal, cytoplasm transitions from blue-stained to pink-stained, cytoplasmic thickness decreases, and nuclei decrease in size and become more condensed.
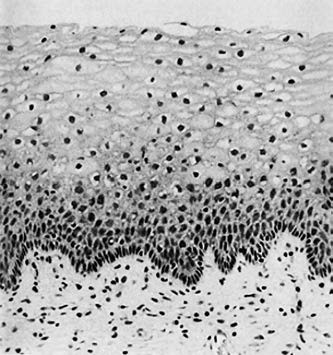
Figure 1 Histology of squamous epithelium
Basal Layer Cells
These correspond to the deep prickle cell layer in histology, consisting of basal and parabasal cell layers.
- Basal Cells: Also known as the germinal layer, they serve as the foundation for squamous epithelium regeneration. Cytologically, these cells are small, round, or oval, with a diameter of 10–12 μm. In Pap staining, the cytoplasm appears blue, and the nuclei are large and round. Basal cells are typically absent in normal vaginal cytological smears from women of reproductive age.
- Parabasal Cells: These cells are round and slightly larger than basal cells, with diameters of 15–30 μm. In Pap staining, their cytoplasm appears light blue, with oval or round nuclei measuring 8–9 μm in diameter. These cells are rarely observed in smears from women of reproductive age with normal ovarian function.
Intermediate Layer Cells
These correspond to the superficial prickle cell layer in histology and are the thickest layer of the squamous epithelium. The cell diameter ranges from 35–50 μm. Cells closer to the basal layer exhibit a boat-shaped morphology, while those nearer to the superficial layer resemble superficial cells in size and shape. The cytoplasm appears lightly blue in Pap staining. Depending on glycogen content, these cells may display basophilic or transparent cytoplasm. Nuclei are small (7–8 μm in diameter), round or oval, lightly stained, with inconspicuous nucleoli.
Superficial Layer Cells
These correspond to the superficial epithelial layer in histology. The cells are large (45–50 μm in diameter), polygonal, with thin, transparent cytoplasm that appears lightly pink or blue in Pap staining. Nuclei are small (5–6 μm in diameter), condensed, and represent the final stage of squamous cell maturation. Superficial cells are the most commonly observed cells in cervical smears from reproductive-age women.
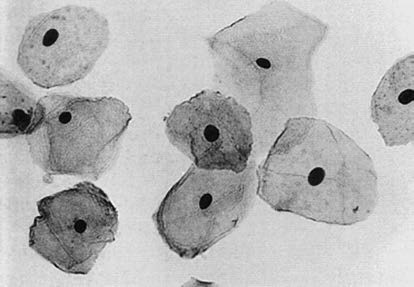
Figure 2 Normal reproductive tract exfoliated cells
Columnar Epithelial Cells
Columnar epithelial cells include cervical mucosal cells and endometrial cells.
Cervical Mucosal Cells
These consist of two types: mucus-secreting cells and ciliated cells. Both types can be observed in cervical smears or brush samples. Mucus-secreting cells are tall columnar or cuboidal, with nuclei located at the base of the cells. The nuclei are slightly larger than those of intermediate squamous cells, with an average diameter of 7–8 μm. They are round or oval, with evenly distributed chromatin and one or more small nucleoli. Cytoplasmic vacuoles are present and readily degrade, often leaving bare nuclei. Ciliated cells are cuboidal or low columnar, with cilia on their apical surface. The nuclei are round or oval and situated at the base of the cells.
Endometrial Cells
These may be observed either singly or in clusters. Endometrial glandular cells are smaller than cervical mucosal cells, with round or oval nuclei that are often eccentric. The nuclear size is similar to or slightly smaller than that of intermediate squamous cell nuclei, with a diameter of 6–7 μm. They exhibit a high nuclear-to-cytoplasmic ratio, dense chromatin, and frequently indiscernible nucleoli. Endometrial cell cytoplasm is sparse, dense, or may exhibit fine vacuoles and basophilic staining. The cell boundaries are unclear.
Non-Epithelial Components
Non-epithelial components observed in smears include macrophages, leukocytes, lymphocytes, and red blood cells.
Application of Reproductive Tract Exfoliated Cells in Gynecological Tumor Diagnosis
Characteristics of Cancer Cells
The changes observed in cancer cells primarily involve alterations in the nucleus, cell morphology, and intercellular relationships.
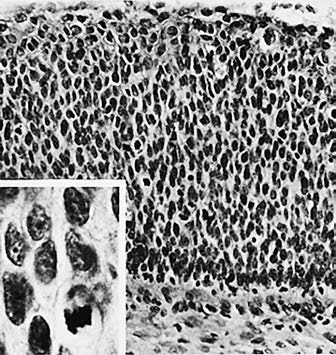
Figure 3 Histology of cervical squamous cell carcinoma
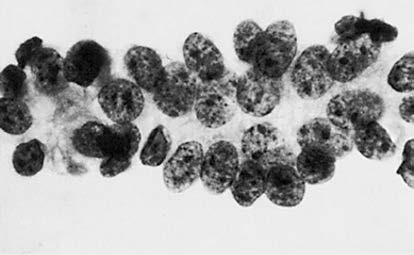
Figure 4 Cytology of cervical squamous cell carcinoma
Nuclear Changes
The nucleus often exhibits enlargement and an abnormal nuclear-to-cytoplasmic ratio. Variations in nuclear size and irregular nuclear shapes are frequently observed. Hyperchromatic nuclei with irregular degrees of staining are common, accompanied by visibly thickened, irregular nuclear membranes. Chromatin displays uneven distribution, may appear coarsely granular or condensed into clumps. Abnormal mitotic figures, nucleolar enlargement, increased numbers of nucleoli, and the presence of abnormal bare nuclei may also be noted.
Cellular Morphological Changes
Cell sizes appear variable, and their shapes lack uniformity. Cytoplasmic volume is reduced, and degenerative processes often result in cytoplasmic vacuolation.
Alterations in Intercellular Relationships
Cancer cells may appear individually or in clusters, often displaying disorganized arrangements. Early-stage cancer smears typically present with a clean, clear background. Smears from advanced-stage cancers tend to exhibit "dirty" backgrounds, characterized by clusters of necrotic cells, red blood cells, and leukocytes.
Reporting Formats for Cervical/Vaginal Cytological Diagnoses
The reporting formats for cervical/vaginal cytology diagnoses primarily include two types: graded diagnosis and descriptive diagnosis.
Papanicolaou Classification for Cervical/Vaginal Cytology
This approach uses a graded diagnostic method, ranging from Papanicolaou classifications I to V. While using grades to indicate the severity of cytological changes seems to suggest distinct boundaries between the classifications, there are no strict objective criteria distinguishing the grades. The distinctions are subject to significant subjective interpretation, and the categories do not align directly with histopathological diagnostic terminology. Over time, the Papanicolaou classification has gradually been replaced by The Bethesda System (TBS).
The Bethesda System (TBS)
The Bethesda System provides a descriptive diagnosis and aligns cytological findings with histopathological terminology while closely integrating them with clinical management. In 1988, the TBS nomenclature system for vaginal cytology was developed in the United States. In 1991, the International Association of Cancer officially adopted the TBS classification for cervical/vaginal cytology reporting. The system underwent revisions in 2001, with the latest version published in 2014, which remains in use. TBS has become the internationally recognized standard guideline for cervical cytological reporting, significantly influencing clinical practice. The Bethesda System introduced three primary improvements: evaluation and reporting of sample adequacy, standardization of diagnostic terminology, and cytopathological diagnosis accompanied by clinical information and pertinent recommendations for medical practitioners. TBS descriptive diagnostic reports generally include the following content:
Sample Adequacy
Satisfactory Samples
Satisfactory samples include an adequate number of well-preserved squamous epithelial cells and specify whether cervical canal cells/transformation zone components are present and whether quality issues, such as interference from blood cells or inflammatory cells, exist. Conventional smears should contain at least 8,000–12,000 identifiable squamous epithelial cells, while liquid-based smears require at least 5,000 identifiable cells. Moreover, any sample containing atypical cells (e.g., atypical squamous cells or atypical glandular cells) is considered satisfactory.
Unsatisfactory Samples
Unsatisfactory samples are reported with the reasons for inadequacy. These may include specimens rejected outright without processing or those processed but insufficient for determining the presence of epithelial abnormalities.
Results
The results are categorized into three main outcomes: no intraepithelial lesions or malignancy, epithelial cell abnormalities, or other findings.
No Intraepithelial Lesions or Malignancy
This category includes findings related to pathogens and other non-neoplastic changes:
- Non-neoplastic cellular changes: These may include squamous metaplasia, keratotic changes, tubal metaplasia, atrophy, or pregnancy-related changes.
- Reactive cellular changes: These may occur due to cervical inflammation, radiation therapy, or the presence of an intrauterine device (IUD).
- Post-hysterectomy glandular cell findings.
- Pathogens: These include Trichomonas vaginalis, fungi (characteristic of Candida species), bacteria, and morphological indications of herpes simplex virus or cytomegalovirus infection.
Other Findings
For women aged 45 or older, the presence of endometrial cells should be documented, even in the absence of squamous intraepithelial lesions.
Epithelial Cell Abnormalities
Squamous Epithelial Cells:
- Atypical Squamous Cells (ASC): These include atypical squamous cells of undetermined significance (ASC-US) and atypical squamous cells—cannot exclude HSIL (ASC-H).
- Squamous Intraepithelial Lesions (SIL): These are further categorized into low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL).
- Squamous Cell Carcinoma.
Glandular Epithelial Cells:
- Atypical Glandular Epithelial Cells: These are categorized as either unspecified or of specific origin, such as endocervical cells, endometrial cells, or glandular cells. Atypical glandular cells suggestive of neoplastic transformation (AGC-FN) include both endocervical glandular cells and other glandular cells.
- Endocervical Adenocarcinoma In Situ.
- Adenocarcinoma: This includes endocervical adenocarcinoma, endometrial adenocarcinoma, extrauterine adenocarcinoma, and adenocarcinoma of unspecified origin.
Other Malignancies
This category encompasses rare tumors originating from the cervix, uterine body, or adnexa, as well as metastatic cancers.
Cervical exfoliative cytology was first applied to cervical cancer screening in the 1940s. This approach significantly reduced the incidence of cervical cancer, as early detection contributed to a decline in cervical cancer mortality rates. It has historically been utilized as a primary screening method for the general population. While it achieves high specificity, reflecting the severity of cervical intraepithelial lesions, its sensitivity is relatively low, with a tendency to miss high-grade lesions.