Assisted reproductive technology (ART) refers to a range of techniques that involve manipulating gametes and embryos outside the body, such as through micromanipulation, to help infertile couples conceive. These methods include artificial insemination, in vitro fertilization and embryo transfer (IVF-ET), as well as other derivative techniques.
Artificial Insemination
Artificial insemination (AI) is a technique in which sperm is introduced into the female reproductive system via non-coital methods to achieve conception. It includes artificial insemination by husband (AIH) and artificial insemination by donor (AID).
Indications for AIH include:
- Male infertility caused by oligospermia, asthenospermia, abnormal liquefaction, sexual dysfunction, or reproductive organ abnormalities.
- Cervical factor infertility.
- Infertility due to reproductive tract abnormalities or psychological factors preventing natural intercourse.
- Immunological infertility.
- Unexplained infertility.
Indications for AID include:
- Irreversible azoospermia, or severe cases of oligospermia, asthenospermia, or teratospermia.
- Unsuccessful vasectomy reversal.
- Ejaculatory disorders.
- Patients falling under indications (1), (2), and (3)—except those with irreversible azoospermia—must be informed by medical professionals that intracytoplasmic sperm injection (ICSI) may provide an opportunity to produce genetically related offspring. If the patient still opts for AID rather than ICSI, informed consent must be signed before the AID procedure can be undertaken.
- Severe hereditary diseases in the male partner and/or family that contraindicate biological reproduction.
- Blood group incompatibility between the mother and fetus, resulting in an inability to deliver a viable newborn.
Under national regulations, all donor sperm used for AID must be sourced from and managed by human sperm banks recognized by the National Health Commission.
Couples experiencing infertility who meet the following criteria may undergo artificial insemination: the presence of normal follicle development, a sufficient number of motile sperm, normal female reproductive tract anatomy, and at least one patent fallopian tube. Based on the insemination site, artificial insemination can be categorized into intrauterine insemination (IUI), intracervical insemination (ICI), and intravaginal insemination (IVI), with IUI being the most commonly used technique in clinical practice.
The standard IUI procedure involves washing and processing the semen to remove seminal plasma, yielding 0.3–0.5 ml of sperm suspension. This suspension is then introduced into the uterine cavity via a catheter through the cervix during the woman's ovulation period. Artificial insemination may be performed in natural menstrual cycles or in ovulation-induced cycles. During ovulation-induced cycles, the number of dominant follicles must be controlled, as the development of three or more dominant follicles may increase the risk of multiple pregnancies. In such cases, it is generally recommended to cancel that cycle's artificial insemination procedure.
In Vitro Fertilization and Embryo Transfer
In vitro fertilization and embryo transfer (IVF-ET) refers to the process of extracting oocytes from the ovary, fertilizing them with sperm outside the body, and culturing the resulting embryos for 3–5 days. Embryos at the cleavage or blastocyst stage are then transferred into the uterine cavity. Commonly known as "test-tube babies," this technique led to the birth of the world's first "test-tube baby" in 1978, as achieved by British researchers Steptoe and Edwards. Edwards was awarded the Nobel Prize for Physiology or Medicine in 2010 for his contributions to this field.
Indications
IVF-ET can be applied to infertility associated with tubal factors, endometriosis, ovulatory disorders, male factor infertility, unexplained infertility, and cervical factors. This procedure is generally considered when pregnancy cannot be achieved using other conventional treatments.
Main Steps
IVF-ET involves ovarian stimulation with medications to promote follicular development and monitoring of follicle growth until maturity. Oocytes are retrieved under transvaginal ultrasound guidance. In vitro fertilization occurs in culture media simulating the fallopian tube environment, with embryos cultivated for 3–5 days to reach the cleavage or blastocyst stage. These embryos are then transferred into the uterine cavity, and luteal phase support therapy is administered simultaneously. Any remaining embryos can be cryopreserved for future use. Full-embryo freezing may also be performed, with embryos thawed and transferred in subsequent menstrual cycles. Two weeks after embryo transfer, pregnancy is confirmed through blood or urine human chorionic gonadotropin (hCG) levels, and an ultrasound at 4–5 weeks post-transfer determines the presence of a clinical intrauterine pregnancy.
Embryo transfer strategies involve complex and individualized decision-making. The "full-embryo freezing" approach refers to freezing all viable embryos for elective frozen embryo transfers. Studies show that frozen embryo transfer increases live birth rates, reduces miscarriage rates, and lowers the risk of severe complications in women with polycystic ovary syndrome (PCOS) compared to fresh embryo transfer. Women with normal ovarian responses but non-ovulatory infertility also benefit from the safety and efficacy of frozen embryo transfer.
Controlled Ovarian Hyperstimulation (COH)
Controlled ovarian hyperstimulation (COH) is the use of medications to induce the simultaneous development and maturation of multiple follicles within a controlled range. This approach increases the number of high-quality oocytes available, thus improving pregnancy rates by providing more transferable embryos. Common COH protocols include the long-agonist protocol, short-agonist protocol, ultra-long protocol, GnRH antagonist protocol, mild stimulation protocol, and protocols under high progesterone conditions, among others.
The selection of a COH protocol emphasizes individualization, taking into account factors such as:
- Female age.
- Treatment objectives.
- Ovarian reserve function.
- Underlying causes of infertility and other pathological conditions.
- Prior medication history.
- Mechanisms of action and costs associated with ovarian stimulation medications.
Complications
Ovarian Hyperstimulation Syndrome (OHSS)
OHSS refers to a pathological condition caused by ovarian stimulation medications, leading to the development of multiple follicles and elevated estrogen levels. This results in increased vascular permeability, fluid accumulation in body cavities, and hemodynamic abnormalities such as hemoconcentration. Elevated hCG levels exacerbate the pathological process. Mild OHSS presents as slight abdominal distension and ovarian enlargement. Moderate to severe OHSS manifests with symptoms such as significant abdominal distension, large volumes of ascites or pleural effusion, hemoconcentration, thrombosis, organ dysfunction, and electrolyte disturbances, which can be life-threatening in severe cases. Approximately 20% of patients undergoing ovarian stimulation experience varying degrees of OHSS, with 1%–4% developing severe cases. High-risk factors include PCOS and elevated anti-Müllerian hormone (AMH) levels. Treatment primarily involves increasing colloid osmotic pressure to expand plasma volume, preventing thrombosis, and providing symptomatic and supportive therapies.
Multiple Pregnancies
The transfer of multiple embryos increases the likelihood of multiple pregnancies. Multiple pregnancies are associated with heightened risks for both the mother and offspring, including miscarriage, preterm birth, low birth weight, gestational hypertension, and postpartum hemorrhage.
A selective single embryo transfer (eSET) is recommended for certain cases, involving cleavage-stage embryos or blastocysts, such as:
- First-time embryo transfers without significant factors affecting pregnancy outcomes.
- Women with uterine conditions unsuitable for twin pregnancies, such as a scarred uterus, uterine malformations, or prior reconstructive surgeries, and those with a history of conditions like cervical insufficiency, twin pregnancies, miscarriage, or preterm birth.
- Women with physical conditions unsuitable for twin pregnancies, such as a height below 150 cm or weight below 40 kg.
- Cases where preimplantation genetic testing reveals suitable embryos for transfer.
- Embryo transfer cycles involving recipients of oocytes obtained through donation.
For cases of multiple pregnancies involving three or more fetuses, selective embryo reduction may be performed during the first or second trimester.
Other Complications
Potential risks during oocyte retrieval include damage to nearby organs such as blood vessels, intestines, bladder, and ureters, which may result in bleeding, infection, or other complications.
A series of related ART techniques have been developed to address infertility caused by various factors, including gamete and embryo cryopreservation, blastocyst culture, intracytoplasmic sperm injection (ICSI), preimplantation genetic testing (PGT), and in vitro maturation (IVM) of oocytes.
Intracytoplasmic Sperm Injection
In 1992, Palermo and colleagues developed a technique where a single sperm cell is injected directly into the cytoplasm of an oocyte, achieving normal fertilization and cleavage. This led to the birth of the first baby conceived through intracytoplasmic sperm injection (ICSI).
Indications
ICSI is indicated for severe oligospermia, asthenospermia, teratozoospermia, irreversible obstructive azoospermia, previous IVF failure, sperm acrosome abnormalities, and couples requiring preimplantation genetic testing (PGT).
Main Steps
The process of ovarian stimulation and follicle monitoring is similar to that in IVF. Oocyte retrieval is performed under transvaginal ultrasound guidance, followed by the removal of cumulus cells. Under a high-magnification inverted microscope, a single sperm is injected into the oocyte cytoplasm. Embryos are cultured in vitro, and subsequent steps, including embryo transfer, luteal support therapy, and the management of complications, are consistent with those in standard IVF-ET protocols.
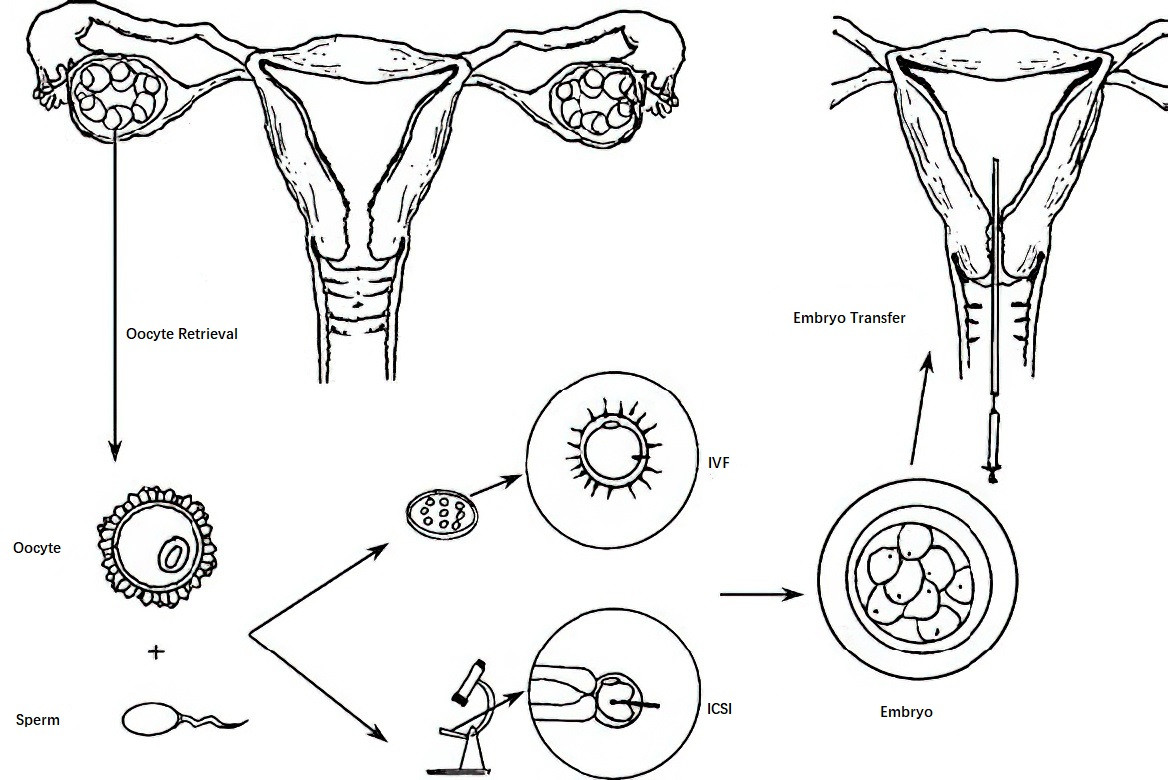
Figure 1 Process of in vitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET)
Preimplantation Genetic Testing
Preimplantation genetic testing (PGT) was first used in 1990 for sex selection in embryos at risk of X-linked genetic disorders. Clinically, it is now primarily applied to the diagnosis of monogenic genetic disorders, chromosomal diseases, sex-linked genetic disorders, and other conditions in high-risk populations that may lead to abnormal offspring.
Procedure
One or two blastomeres are retrieved from embryos on day 3 of in vitro culture or a small number of trophectoderm cells are biopsied from blastocysts on day 5. These cells undergo cellular and/or molecular genetic testing to screen for chromosomal abnormalities or disease-causing genetic mutations. Only embryos with normal karyotypes and/or genotypes are selected for transfer to achieve healthy offspring. This technique enables prenatal diagnosis to be shifted to the pre-implantation stage, avoiding harm associated with mid-trimester pregnancy termination following conventional prenatal diagnostics.
Advances in cellular and molecular biology have led to the use of high-throughput microarray and next-generation sequencing technologies in clinical practice. These methods facilitate the diagnosis of hundreds of single-gene disorders and chromosomal abnormalities in the embryonic stage.
Categories of PGT
Preimplantation Genetic Testing for Monogenic Disorders (PGT-M):
PGT-M is used to detect specific pathogenic genetic mutations, including:
- Single-gene disorders such as autosomal dominant/recessive disorders and X/Y-linked conditions.
- Genetically predisposed severe diseases.
- Human leukocyte antigen (HLA) typing.
Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR)
PGT-SR is suitable for individuals or couples with structural chromosomal abnormalities, including reciprocal translocations, Robertsonian translocations, inversions, complex rearrangements, and pathogenic microdeletions or microduplications. Advanced molecular diagnostic techniques allow the differentiation between embryos that carry balanced translocations and those with normal chromosomal structures.
Preimplantation Genetic Testing for Aneuploidy (PGT-A)
PGT-A detects chromosomal aneuploidies in embryos prior to transfer. It is often used in women of advanced maternal age, individuals with unexplained recurrent pregnancy loss, and other cases to improve live birth rates and reduce the risk of early miscarriages.
Contraindications for PGT:
- Genetic disorders with unclear or unidentified gene mutations.
- Selection for traits unrelated to diseases, such as gender, appearance, height, or skin color.
- Situations violating local laws or ethical norms.
Gamete Intrauterine Transfer
Gamete intrauterine transfer (GIUT) involves retrieving male and female gametes, processing them in vitro, and transferring them into the female reproductive system. Among the various types of gamete transfer techniques, intrauterine transfer via the transvaginal route has seen relatively widespread use. This method is technically simple and is primarily indicated for individuals with bilateral tubal obstruction, absence, or dysfunction. However, as in vitro culture techniques have continued to improve, clinical application of gamete transfer techniques has declined. At present, GIUT serves as an alternative option mainly for patients with limited financial resources or those who have experienced repeated IVF-ET failures.
Application of Assisted Reproductive Technologies in Fertility Preservation for Women
Chemotherapy, radiotherapy, and surgical procedures involving reproductive organs can significantly impair or completely eliminate fertility in cancer patients. Consequently, there is a growing demand among young cancer patients for fertility preservation. Prior to cancer treatment, ART-based fertility preservation methods such as embryo freezing, oocyte freezing, and ovarian tissue freezing are available for female cancer patients.
Ethics and Management
Given its implications for offspring health and human reproduction, assisted reproductive technology (ART) requires stringent ethical, moral, and legal oversight as part of routine management. The rapid development and proliferation of new ART techniques, such as cytoplasmic transfer, nuclear transfer, therapeutic cloning, and in vitro differentiation of embryonic stem cells, underscore the need for enhanced ethical and regulatory supervision.