Polycystic ovary syndrome (PCOS) is one of the most common gynecological endocrine disorders. It is clinically characterized by infrequent ovulation or anovulation, clinical or biochemical manifestations of hyperandrogenism, and polycystic ovarian morphology. PCOS is frequently associated with insulin resistance and obesity. First described by Stein and Leventhal in 1935, it is also known as Stein-Leventhal syndrome.
Associated Factors
The exact cause of PCOS remains unclear, but it is believed to result from the combined influence of genetic and environmental factors.
Genetic Factors
PCOS exhibits familial clustering, though its specific genetic mechanisms are not well understood. Through genome-wide association studies (GWAS), 11 PCOS-related susceptibility genes—such as LHCGR, DENND1A, and THADA—have been identified. Additional susceptibility genes have subsequently been reported in European populations.
Environmental Factors
In utero exposures can influence the risk of PCOS in offspring during adulthood. Studies suggest that exposure to elevated androgen or anti-Müllerian hormone (AMH) levels during pregnancy may predispose female offspring to PCOS. Postnatal metabolic abnormalities, such as obesity or hyperinsulinemia, further increase the risk of developing PCOS.
Pathophysiology and Endocrine Characteristics
The primary endocrine features of PCOS include:
- Hyperandrogenism;
- Excess estrogen (particularly estrone);
- An increased luteinizing hormone/follicle-stimulating hormone (LH/FSH) ratio;
- Hyperinsulinemia.
These changes are thought to arise from the following mechanisms:
Dysfunction of the Hypothalamic-Pituitary-Ovarian (HPO) Axis
Increased pituitary sensitivity to gonadotropin-releasing hormone (GnRH) leads to excessive LH secretion. Elevated LH stimulates excess androgen production by ovarian stromal and theca cells. High intrafollicular androgen levels inhibit follicular maturation, preventing the formation of dominant follicles. However, immature follicles still secrete estradiol (E2) at levels equivalent to those of the early follicular phase. Additionally, androstenedione is converted to estrone (E1) in peripheral tissues via aromatase, resulting in hyperestrogenism.
Continuous estrone secretion, combined with persistent estradiol levels, exerts a positive feedback effect on the hypothalamus and pituitary, leading to elevated LH secretion with increased amplitude and frequency. This disrupts the cyclical nature of the menstrual cycle, eliminating the mid-cycle LH surge required for ovulation. Concurrently, estrogen exerts negative feedback on FSH secretion, resulting in relatively low FSH levels and an elevated LH/FSH ratio. A lack of FSH causes arrested follicular development, preventing dominant follicle formation and leading to a cycle of hyperandrogenism and anovulation. This creates a self-reinforcing pathological cycle, ultimately resulting in polycystic ovarian morphology.
Insulin Resistance and Hyperinsulinemia
Insulin resistance (IR) refers to a state in which peripheral tissues are less sensitive to insulin, reducing its biological effectiveness. Approximately 50% of patients with PCOS have some degree of insulin resistance and compensatory hyperinsulinemia. Elevated insulin levels act on pituitary insulin receptors, amplifying LH release and stimulating androgen production by the ovaries and adrenal glands. Hyperinsulinemia also suppresses the hepatic synthesis of sex hormone-binding globulin (SHBG), leading to increased levels of free testosterone.
Adrenal Endocrine Dysfunction
Some PCOS patients exhibit elevated levels of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS), which may result from increased activity of the adrenal zona reticularis enzyme P450c17α, heightened sensitivity of adrenal cells to adrenocorticotropic hormone (ACTH), or adrenal hyperfunction. Elevated DHEAS levels indicate that excessive androgen production originates from the adrenal glands.
Pathology
Ovarian Morphology
Bilateral ovarian enlargement occurs, with ovary size increasing 2–5 times compared to those of normal reproductive-aged women. Gross examination reveals grayish-white, thickened, and firm ovarian capsules. Cross-sectional views demonstrate uniformly thickened ovarian tunica albuginea, which is 2–4 times thicker than normal. Subcapsular regions contain ≥12 follicles, each measuring 2–9 mm in diameter.
Microscopic findings include thickened, sclerotic tunica albuginea, cortical fibrosis, sparse cellularity, and prominent vasculature. Multiple cystically dilated follicles and atretic follicles are observed beneath the thickened capsule, with no evidence of mature follicle development or ovulation.
Endometrial Changes
Infrequent or absent ovulation leads to prolonged unopposed estrogen exposure, resulting in varying degrees of endometrial hyperplasia in PCOS patients. This increases the risk of developing atypical hyperplasia and endometrial cancer.
Clinical Manifestations
PCOS typically begins during puberty. The main clinical manifestations include menstrual irregularities, infertility, hyperandrogenism, and obesity.
Menstrual Irregularities
Menstrual irregularities are the most common symptom. These often present as oligomenorrhea (cycles ranging from 35 days to 6 months) or amenorrhea, often preceded by reduced menstrual flow or oligomenorrhea. Some individuals may experience irregular uterine bleeding, frequent menstruation, or unpredictable bleeding patterns.
Infertility
Infertility is a common issue for women of reproductive age with PCOS, primarily due to ovulatory dysfunction.
Hirsutism and Acne
Hirsutism and acne are the most frequent clinical manifestations of hyperandrogenemia. Hirsutism typically involves sexual hair, with a dense pubic region resembling a male pattern, extending to the perianal area, groin, or the midline of the abdomen. Some individuals may also develop fine hair on the upper lip, chin, or around the areolae. Oily skin and acne are prevalent and are associated with androgen-induced stimulation of sebaceous glands.
Obesity
A higher proportion of women with PCOS meet the criteria for obesity (BMI ≥28 kg/m2), primarily presenting with abdominal obesity (waist circumference ≥85 cm or waist-to-hip ratio ≥0.85). Obesity is associated with insulin resistance, hyperandrogenism, and leptin resistance.
Acanthosis Nigricans
Skin folds in areas such as the labia, posterior neck, axillae, submammary regions, and groin often exhibit gray-brown hyperpigmentation. The affected skin becomes symmetrically thickened with a soft texture.
Auxiliary Examinations
Basal Body Temperature Monitoring
Basal body temperature typically shows a monophasic curve.
Ultrasound Imaging
Ovarian enlargement is observed, with increased echogenicity of the ovarian capsule and a smooth contour. Enhanced stromal echogenicity is also present. One or both ovaries may show ≥12 anechoic follicles with diameters of 2–9 mm, arranged peripherally in a "string of pearls" pattern.
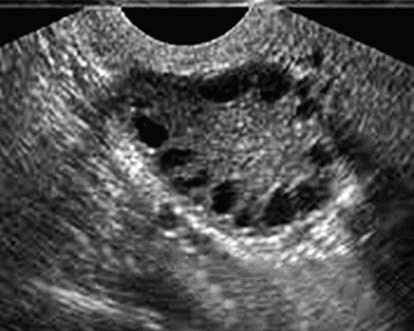
Figure 1 Ultrasound imaging of PCOS ("string of pearls" sign)
Polycystic ovary morphology (PCOM) is defined as ≥12 follicles measuring 2–9 mm in diameter in one or both ovaries and/or an ovarian volume ≥10 mL, as identified on ultrasound. However, PCOM can also be observed in non-PCOS individuals; 20–30% of healthy reproductive-aged women may exhibit PCOM, with higher prevalence during adolescence. Therefore, PCOM alone is not sufficient for diagnosing PCOS. Ovulatory monitoring in individuals with PCOS often shows a lack of dominant follicle development or ovulation, or infrequent ovulation.
Laboratory Tests
Serum Androgens
Mildly elevated or normal serum testosterone levels are observed, typically no more than twice the upper limit of the normal range. Androstenedione levels are elevated, while DHEA and DHEAS levels are normal or slightly elevated.
Serum FSH and LH
Serum FSH levels are normal or slightly low. Approximately 50% of individuals exhibit elevated serum LH levels, although no pre-ovulatory LH surge is observed. An LH/FSH ratio ≥2 is common in non-obese PCOS individuals. In obese patients, this ratio may fall within the normal range due to leptin-induced suppression of central LH release.
Anti-Müllerian Hormone (AMH)
Serum AMH levels are 2–4 times higher than those of age-matched controls. However, AMH alone is not currently recommended for PCOS diagnosis.
Serum Prolactin (PRL)
20–35% of PCOS patients exhibit mildly elevated serum PRL levels.
Metabolic Assessments
Initial assessments typically include fasting blood glucose and an oral glucose tolerance test (OGTT). Fasting insulin levels and post-glucose challenge insulin levels may be measured to evaluate insulin resistance. Additional evaluations may include lipid profiles and liver function tests.
Additional Tests
Thyroid function, 17α-hydroxyprogesterone (17-OHP), and sex hormone-binding globulin (SHBG) levels may be assessed as needed.
Diagnostic Curettage
Currettage may be performed in cases of amenorrhea or irregular menstruation to assess the degree of endometrial hyperplasia. This procedure is now infrequently used in clinical practice.
Diagnosis
Due to the heterogeneity in clinical phenotypes, there is some debate regarding the diagnostic criteria for PCOS. Internationally, several criteria have been proposed, including the National Institutes of Health (NIH) criteria, the Rotterdam criteria, and the Androgen Excess and PCOS Society (AES) criteria. Among these, the Rotterdam criteria are more commonly used, which include:
- Oligo-ovulation or anovulation;
- Clinical and/or biochemical evidence of hyperandrogenism;
- Polycystic ovary morphology (PCOM);
A diagnosis requires the presence of at least 2 out of the 3 criteria, with other conditions causing hyperandrogenism or ovulatory dysfunction excluded.
Differential Diagnosis
Thecoma or Hyperplasia of the Theca Cells
This condition exhibits clinical and endocrine features similar to PCOS but is more severe. Serum testosterone levels are elevated, while DHEAS levels remain normal, and the LH/FSH ratio may fall within the normal range. Ovarian biopsy shows clusters of luteinized theca cells in the ovarian cortex, without the appearance of multiple small follicles characteristic of PCOM in the subcortex.
Adrenal Cortical Hyperplasia or Tumors
When serum DHEAS levels exceed twice the upper limit of normal, adrenal cortical hyperplasia or tumors should be considered. Adrenal cortical hyperplasia is characterized by markedly elevated serum 17α-hydroxyprogesterone (17α-OHP), heightened responsiveness to ACTH stimulation tests, and a dexamethasone suppression test inhibition rate of ≤0.70. Patients with adrenal cortical tumors do not exhibit significant responses to these tests.
Androgen-Secreting Ovarian Tumors
Tumors such as ovarian Sertoli-Leydig cell tumors or ovarian hilus cell tumors can produce androgens. These are typically unilateral, solid tumors. Ultrasound, CT, or MRI imaging can assist in the diagnosis.
Other Conditions
Significantly elevated prolactin levels warrant consideration of pituitary prolactinomas.
Treatment
Recent studies have shown that the management of PCOS involves addressing not only menstrual irregularities and infertility but also metabolic disorders, cardiovascular diseases, cancers, and mood disorders. Comprehensive management of these chronic conditions is necessary.
Lifestyle Modification
Lifestyle modification is the first-line treatment for PCOS. For obese individuals with PCOS, incorporating scientifically informed dietary practices and appropriate physical activity is encouraged to reduce weight and alleviate central obesity. Improvements in insulin sensitivity and reductions in insulin and testosterone levels may occur as a result, potentially restoring ovulation and fertility.
Pharmacological Therapy
Regulation of Menstrual Cycles
Managing menstrual cycles through regular and appropriate medication use helps protect the endometrium, in addition to addressing irregularities.
Combined Short-Acting Oral Contraceptives
Combinations of estrogen and progestin provide a key option. Progestin inhibits excessive secretion of pituitary LH through negative feedback, reducing ovarian androgen production and directly acting on the endometrium to prevent hyperplasia and regulate menstrual cycles. Estrogen increases hepatic production of sex hormone-binding globulin (SHBG), reducing free testosterone levels. Beyond cycle regulation, these agents effectively treat hirsutism and acne and are suitable for reproductive-aged PCOS women with hyperandrogenic symptoms who do not have immediate childbearing needs. Adolescents require a careful risk-benefit assessment. Caution is advised in perimenopausal women and those with obesity, a smoking history, hypertension, diabetes, or coagulopathy.
Second-Half Cycle Progestin Therapy
This approach regulates menstrual cycles and protects the endometrium. It may improve elevated LH levels through negative feedback, potentially restoring ovulation. This therapy can serve as a first-line option for adolescents, perimenopausal women, or patients planning pregnancy, but it is less effective in managing hyperandrogenic symptoms.
Sequential Estrogen-Progestin Therapy
This treatment suits a small subset of PCOS patients with thin endometrium caused by endogenous estrogen deficiency, as well as those experiencing perimenopausal PCOS symptoms.
Reduction of Androgen Levels
Combined Short-Acting Oral Contraceptives
These serve as the first-line treatment for PCOS patients exhibiting hirsutism, acne, or hyperandrogenemia. Formulations containing cyproterone acetate or drospirenone are often used. Improvements in acne typically take 3–6 months, while hirsutism may require 6–12 months of treatment.
Spironolactone
As a competitive antagonist of aldosterone, spironolactone reduces ovarian and adrenal androgen synthesis, promotes androgen degradation, and competes with androgen receptors in hair follicles. It may be used when oral contraceptives are contraindicated, poorly tolerated, or ineffective. The standard dosage is 40–100 mg/day, and effects usually take at least 6 months to emerge. As potassium-sparing diuretics, spironolactone use requires monitoring of serum potassium levels.
Glucocorticoids
Glucocorticoids are appropriate for managing androgen excess stemming from adrenal or mixed adrenal and ovarian sources. Prednisone or dexamethasone may be prescribed, effectively reducing DHEAS levels. Prolonged use requires caution as it may lead to adverse effects across multiple organ systems.
Improvement of Insulin Resistance
Insulin sensitizers are commonly used in obese patients or those with insulin resistance. Metformin is the preferred drug as it inhibits hepatic glucose production and enhances peripheral tissue insulin sensitivity. By lowering serum insulin levels, it helps correct hyperandrogenism, improves ovarian ovulation function, and enhances the efficacy of ovulation induction treatments. The common dosage involves 500 mg orally per dose, 2–3 times daily.
Ovulation Induction
For patients seeking pregnancy, ovulation induction treatments may be initiated following basic treatments such as lifestyle modification, anti-androgen therapy, and insulin resistance management. Vigilance is required to monitor and address ovarian hyperstimulation syndrome (OHSS).
Clomiphene Citrate (CC)
As a conventional first-line ovulation induction medication, it exhibits weak anti-estrogenic effects, competing with endogenous estrogen receptors in the hypothalamus and pituitary. This relieves estrogenic suppression of gonadotropin secretion, thus promoting ovulation. CC therapy begins on days 2–5 of the menstrual or withdrawal bleeding cycle, with an initial dosage of 50 mg/day for 5 days. Dosage can be incrementally increased to a maximum of 150 mg/day per cycle if ovulation does not occur.
Letrozole (LE)
Letrozole is an alternative first-line agent for ovulation induction in PCOS and may be used for CC-resistant cases. Treatment begins on days 2–5 of the menstrual or withdrawal cycle at 2.5 mg/day for 5 days. Dosages can be increased to 5.0–7.5 mg/day in subsequent cycles if ovulation is not achieved.
Gonadotropins
Gonadotropins may serve as second-line ovulation induction therapies, either alone or in combination with CC or LE. The incidence of OHSS is higher when using gonadotropins compared to CC or LE alone.
Laparoscopic Ovarian Drilling (LOD)
LOD is not a routine treatment and is typically performed during laparoscopic surgery for other conditions. It is effective in patients with elevated LH and free testosterone levels. The mechanism involves destroying androgen-producing ovarian stroma, indirectly regulating the hypothalamic-pituitary-ovarian axis, lowering serum LH and testosterone levels, and improving pregnancy rates. Risks such as pelvic adhesions and ovarian insufficiency following LOD should be considered.
In Vitro Fertilization and Embryo Transfer (IVF-ET)
IVF-ET can be an option for patients who fail to conceive after other treatments or who have additional infertility factors, such as tubal obstruction or severe male factor infertility.
Psychological Therapy
PCOS patients, due to concerns about body image, irregular menstruation, and fertility issues, may experience anxiety or depression. Effective communication during diagnosis and treatment, addressing both physical and emotional health, and safeguarding patient privacy are essential. Positive guidance is beneficial, and referral to a psychological medicine department may be indicated for further intervention when necessary.