Precocious puberty is defined as the appearance of secondary sexual characteristics that occur earlier than the mean age of pubertal development for a population, by more than 2 standard deviations. In clinical practice, precocious puberty in females is diagnosed when breast development occurs before the age of 7.5 years or menarche occurs before the age of 10 years.
Etiology and Classification
Based on whether the hypothalamic-pituitary-gonadal (HPG) axis is prematurely activated, precocious puberty can be classified into central precocious puberty, peripheral precocious puberty, and incomplete precocious puberty.
Central Precocious Puberty (CPP)
This is also referred to as GnRH-dependent precocious puberty, complete precocious puberty, or true precocious puberty. CPP is further divided into idiopathic precocious puberty and secondary precocious puberty. It is caused by the premature activation of the HPG axis, which leads to the early maturation of secondary sexual characteristics. CPP accounts for approximately 80% of cases of precocious puberty in girls.
Idiopathic Precocious Puberty
This diagnosis applies when a thorough examination reveals no underlying organic cause for the early onset of puberty. It is characterized by progressive maturation of secondary sexual characteristics. Estradiol and gonadotropin levels reach pubertal levels. Bone age advancement is significant, and compared to peers, growth in height and weight is markedly accelerated. However, early epiphyseal fusion results in short final adult stature despite early growth acceleration.
Secondary Precocious Puberty
This form is secondary to central nervous system (CNS) abnormalities or peripheral precocious puberty. Various CNS disorders can lead to or coexist with CPP, including hypothalamic tumors such as gliomas, hamartomas, astrocytomas, craniopharyngiomas, as well as non-tumorous conditions like encephalitis, meningitis, hydrocephalus, or head trauma. Certain metabolic conditions, such as primary hypothyroidism, can also result in CPP. Elevated hypothalamic secretion of thyrotropin-releasing hormone (TRH) in primary hypothyroidism increases both thyroid-stimulating hormone (TSH) and gonadotropin secretion, contributing to precocious puberty.
Peripheral Precocious Puberty (PPP)
This is also called GnRH-independent precocious puberty or pseudoprecocious puberty. Its defining characteristic is that the hormonal stimulation for premature development of secondary sexual characteristics does not originate from the activation of the HPG axis. Instead, it results from excessive secretion of sex steroids by the gonads or adrenal glands, or from overexposure to exogenous estrogens. Additionally, it may eventually lead to the premature activation of the HPG axis, subsequently triggering central precocious puberty.
PPP is further classified into isosexual precocious puberty and contrasexual precocious puberty:
Isosexual precocious puberty involves the development of secondary sexual characteristics consistent with the individual's biological sex. Causes include estrogen-secreting ovarian cysts, ovarian tumors, adrenal cortical tumors, prolonged exposure to exogenous estrogens, and conditions such as McCune-Albright syndrome.
Contrasexual precocious puberty involves the development of sexual characteristics opposite to the individual's biological sex, often caused by androgen-secreting diseases or tumors. In females, congenital adrenal hyperplasia (CAH) is a common cause of contrasexual precocious puberty.
Ovarian Cysts
Functional ovarian cysts are the most common cause of peripheral precocious puberty in girls. These cysts secrete varying amounts of estrogen, leading to precocious puberty.
Ovarian Tumors
Hormone-secreting ovarian tumors can cause precocious puberty. Granulosa cell tumors are the most common type, typically associated with isosexual precocious puberty. Sertoli-Leydig cell tumors, simple stromal tumors, and gonadoblastomas may produce androgens, resulting in contrasexual precocious puberty.
Exogenous Sex Hormones
Estrogen-containing substances found in medications, foods, or cosmetics may result in precocious puberty following exposure.
McCune-Albright Syndrome
This congenital disorder is caused by activating mutations in the GNAS1 gene on chromosome 20. Clinical features include a classic triad of peripheral precocious puberty, polyostotic fibrous dysplasia of the bone, and café-au-lait spots on the skin.
Incomplete Precocious Puberty
This is also known as partial precocious puberty or variant pubertal development. This type involves isolated premature development of specific secondary sexual characteristics, such as early adrenarche, isolated pubarche, isolated premature breast development, or premature menarche. The development of sexual characteristics is generally self-limiting. These patients do not exhibit typical growth acceleration or skeletal maturation, and hormone levels remain appropriate for their age.
Diagnosis and Differential Diagnosis
A comprehensive diagnosis requires consideration of the age of secondary sexual characteristic onset, medical history, clinical presentation, reproductive hormone levels, and imaging evaluations. The diagnostic process includes determining whether precocious puberty is isosexual or contrasexual, identifying whether it is central, peripheral, or incomplete, and confirming the specific cause.
Medical History
Key aspects include detailed inquiry into birth history, including potential birth injuries or asphyxia; history of fever, convulsions, or epilepsy during infancy; history of head trauma or surgeries. Attention is paid to the age and progression of secondary sexual characteristic development, as well as growth patterns in height and weight. Information is collected on accidental ingestion of endocrine medications or exposure to hormone-containing substances or foods, and on any tumors affecting the brain, ovaries, or adrenal glands.
Physical Examination
Key indicators include height, weight, and the development of the breasts and internal and external genitalia. A pelvic and abdominal examination is conducted to assess for masses. In central precocious puberty (CPP), growth spurts occur early, often leading to height and weight exceeding more than 2 standard deviations above the mean for age-matched peers. In peripheral precocious puberty (PPP), height and weight are generally consistent with age. Sexual development can be assessed according to the Tanner staging system. General physical examination involves noting signs such as skin pigmentation, hypothyroid-related features, neurological abnormalities, or androgenization.
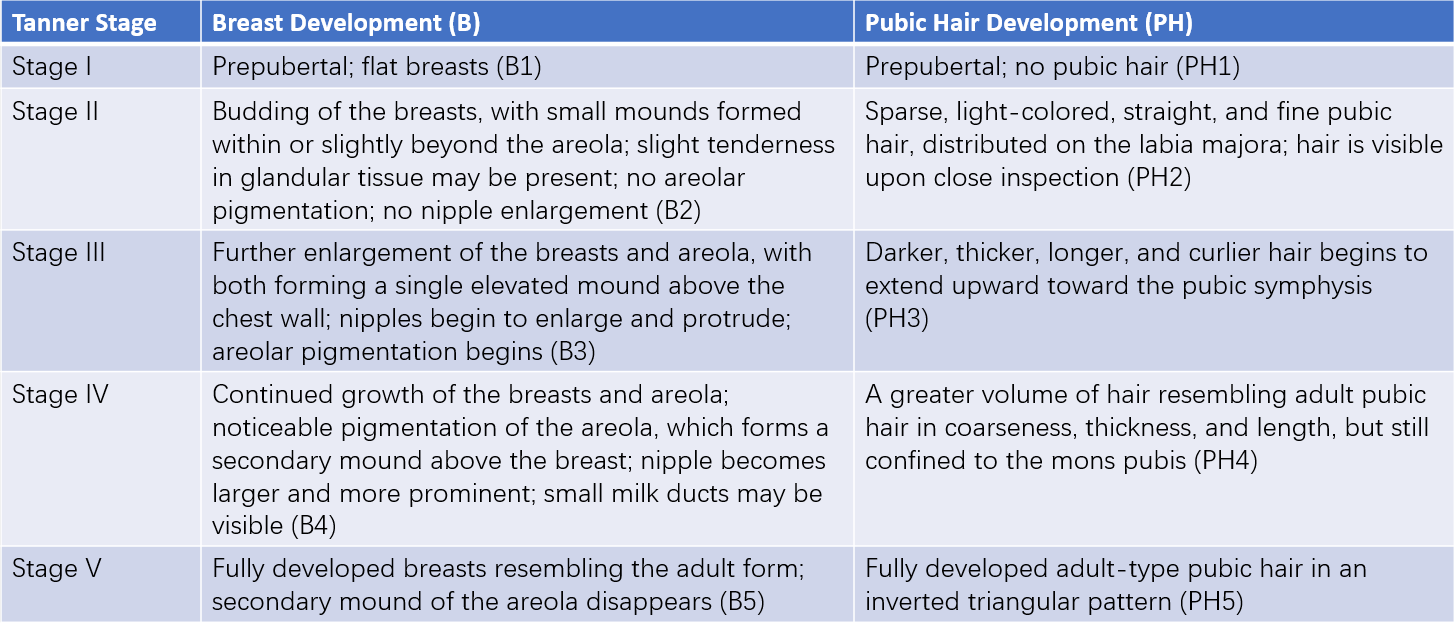
Table 1 Stages of secondary sexual characteristic maturity
Auxiliary Examinations
Hormone Testing
Serum Reproductive Hormone Levels
Elevated luteinizing hormone (LH) serves as a key biochemical indicator of HPG axis activation. However, its diagnostic utility is limited due to its pulsatile secretion, diurnal rhythm, and variation across Tanner stages.
GnRH Stimulation Test
This test can confirm CPP in cases where baseline LH levels are unstable.
- Method: GnRH is administered at 2.5 μg/kg per dose, up to a maximum dose of 100 μg/dose, via subcutaneous or intravenous injection. Blood samples are taken at 0, 30, 60, and 90 minutes post-injection to measure serum LH and follicle-stimulating hormone (FSH) levels.
- Interpretation: An LH peak >5 IU/L or an LH/FSH peak ratio >0.6 supports a diagnosis of CPP.
TSH, T3, and T4 Testing
These are essential for evaluating thyroid function.
Additional Tests
For suspected adrenal pathology, tests for cortisol, 11-deoxycortisol, and 17α-hydroxyprogesterone are recommended.
Bone Development Assessment
Indicators such as bone age, bone mineral content, and bone density are assessed.
Imaging Studies
Gynecological and abdominal ultrasounds, as well as head CT or MRI, can be used to identify abnormalities in related regions. In female patients, gynecological ultrasound findings of a uterine length between 3.4 and 4.0 cm and ovarian volume between 1 and 3 mL (calculated as length × width × height × 0.5233), with multiple follicles ≥4 mm in diameter, suggest pubertal onset.
Treatment
The treatment approach involves addressing the underlying cause, controlling and slowing the progression and maturation of secondary sexual characteristics, restoring psychological and behavioral outcomes appropriate for age, and improving final adult height.
Addressing the Underlying Cause
In cases caused by tumors in the CNS or gonads, treatment options such as radiation or surgery are applied. Thyroid hormone supplementation is used for hypothyroidism. For cases caused by exposure to hormone-containing medications, foods, or cosmetics, removing the source of exposure is critical.
Observation and Monitoring
This approach is appropriate for slow-progressing cases of precocious puberty where the impact on adult height is negligible. For example, if bone age advancement is present but growth velocity remains rapid and projected adult height is unaffected, ongoing observation may suffice. Pubertal development is a dynamic process and requires periodic monitoring and evaluation to decide if and when intervention becomes necessary.
Pharmacological Treatment
GnRH Agonist (GnRH-a) Therapy
Indications for Treatment
The indications include:
- Rapidly progressing CPP with significant skeletal maturation and accelerated secondary sexual characteristic development that exceeds the rate of linear growth.
- Impairment of final adult height.
- Psychological or behavioral disturbances.
Treatment Protocol
The standard initial dose of GnRH-a is 3.75 mg, with subsequent adjustments to 80–100 μg/kg every 4 weeks. Alternatively, a standardized dose of 3.75 mg can be administered every 4 weeks. Discontinuation is influenced by factors such as satisfactory height outcomes, quality of life considerations, and synchronization of pubertal development with age-matched peers, although fixed criteria for discontinuation are lacking. During therapy, regular monitoring of sexual development, growth velocity, and bone age is essential to ensure appropriate dosing and intervals.
Thyroid Hormone Replacement Therapy
This is a treatment for hypothyroidism-induced precocious puberty.
Adrenal Corticosteroid Replacement Therapy
Lifelong treatment is necessary for congenital adrenal hyperplasia (CAH).
Psychological, Health, and Sexual Education
Girls with precocious puberty often experience psychological distress, such as shyness or low self-esteem, due to pubertal development occurring earlier than their peers. Parental anxiety is also common. Disseminating necessary medical knowledge is beneficial for guiding patients and their families toward active treatment and improving outcomes, while reducing psychological impact. In addition, since ovulation is possible, girls with precocious puberty have reproductive potential, necessitating measures to prevent inappropriate sexual behavior or harm from external violence.