Gestational trophoblastic neoplasia arises in 50% of cases following molar pregnancy, while the remaining cases originate from miscarriage, full-term pregnancy, or ectopic pregnancy. All cases of invasive mole are secondary to molar pregnancy. Choriocarcinoma can develop after both molar and non-molar pregnancies. Invasive mole exhibits lower malignancy and generally has a better prognosis compared to choriocarcinoma. Choriocarcinoma, however, is highly malignant, with early and widespread metastases. Before the advent of chemotherapy, its mortality rate exceeded 90%. Advancements in diagnostic techniques and chemotherapy have significantly improved the prognosis.
Pathology
Macroscopic examination of invasive mole reveals vesicular tissue infiltrating the uterine myometrium. When the lesion approaches the uterine serosa, purplish-blue nodules can be seen on the uterine surface. The lesion may also penetrate the serosa or invade the broad ligament. Microscopic findings show hydropic villi within the myometrium or blood vessels, along with trophoblastic hyperplasia and atypia. However, villous structures may degenerate, leaving only villous remnants.
For choriocarcinoma, macroscopic findings include tumors located within the uterine myometrium that may protrude into the uterine cavity or penetrate the serosa. These tumors vary in size and shape, lack defined borders with surrounding tissues, have a soft and fragile texture, exhibit a sponge-like appearance, dark red color, and are accompanied by significant hemorrhage and necrosis. Microscopic examination reveals tumor cells composed of cytotrophoblasts, syncytiotrophoblasts, and intermediate trophoblasts, forming sheets of cells. The cells exhibit marked atypia, frequent mitoses, and lack chorionic villous structures. The tumor is devoid of inherent stroma and vasculature, leading to extensive invasion of the myometrial blood vessels and causing significant hemorrhage and necrosis.
Clinical Manifestations
Non-Metastatic Trophoblastic Tumors
These are most commonly secondary to molar pregnancy.
Vaginal Bleeding
Persistent irregular bleeding of variable volume occurs after uterine evacuation of molar pregnancy, miscarriage, or full-term delivery. Bleeding may also present after a period of normal menstruation followed by secondary amenorrhea, then another episode of vaginal bleeding. Prolonged bleeding may lead to anemia.
Uterine Subinvolution or Irregular Enlargement
The uterus often fails to return to its normal size 4 to 6 weeks after molar evacuation, presenting with a softer texture. Lesion size and location within the myometrium may result in irregular uterine enlargement.
Theca-Lutein Ovarian Cysts
Persistent bilateral or unilateral theca-lutein cysts may occur due to sustained hCG elevation after molar evacuation, miscarriage, or full-term delivery.
Abdominal Pain
While generally absent, acute abdominal pain and intraperitoneal bleeding can occur if the uterine lesion penetrates the serosa. Lesion necrosis with secondary infection may lead to abdominal pain and purulent vaginal discharge. Torsion or rupture of theca-lutein ovarian cysts may also cause acute abdominal pain.
Pseudopregnancy Symptoms
Breast enlargement, darkened nipples and areolas, possible secretion of colostrum-like fluid, hyperpigmentation of the vulva, vagina, and cervix, as well as softening of the reproductive tract tissues, may occur due to hCG and elevated estrogen/progesterone levels.
Metastatic Trophoblastic Tumors
These commonly follow non-molar pregnancies and metastasize primarily via hematogenous spread, with early and widespread dissemination. The most frequent metastatic sites include the lungs (80%), followed by the vagina (30%), pelvis (20%), liver (10%), and brain (10%). Localized bleeding is a characteristic symptom across metastatic sites.
Metastatic trophoblastic tumors may present with symptoms of both the primary and metastatic lesions, though the primary lesion may also resolve while the metastatic lesion continues to progress, presenting solely with metastatic symptoms and potentially leading to misdiagnosis.
Lung Metastases
Symptoms may be absent and only detected through chest X-rays or CT scans. Typical manifestations include chest pain, coughing, hemoptysis, and dyspnea, which may occur acutely or persist chronically. In rare cases, pulmonary infarction can result from trophoblastic emboli in the pulmonary arteries, causing acute pulmonary hypertension, acute respiratory failure, and right heart failure.
Vaginal Metastases
Lesions are often located on the anterior vaginal wall or vaginal fornix and appear as purplish-blue nodules. Lesion rupture causes irregular vaginal bleeding or significant hemorrhage. Retrograde metastasis through the uterine venous plexus is considered the underlying mechanism.
Liver Metastases
Liver involvement is a poor prognostic factor and often occurs alongside lung metastases. Small lesions may be asymptomatic, while larger lesions can cause pain in the right upper abdomen or liver region, jaundice, or, in the case of a ruptured lesion with capsular perforation, intraperitoneal bleeding and rapid death.
Brain Metastases
Brain involvement carries a grim prognosis and is a leading cause of death. It often occurs with pulmonary and/or vaginal metastases. Initial brain metastases may be asymptomatic. Lesion progression can be divided into three stages:
- Tumor Embolization Stage: Temporary ischemic symptoms such as sudden falls, transient aphasia, or blindness may occur.
- Brain Tumor Stage: Lesion growth into brain tissue results in headaches, projectile vomiting, hemiplegia, seizures, and eventual coma.
- Brain Herniation Stage: Expansion of tumors and surrounding tissue edema elevate intracranial pressure, causing brain herniation, compression of vital centers, and death.
Other Metastases
Additional metastatic sites include the spleen, kidneys, bladder, gastrointestinal tract, and bones, with symptoms varying according to the affected organ.
Diagnosis
Clinical Diagnosis
Serum hCG Measurement
Abnormal hCG levels serve as the primary diagnostic criterion. Imaging evidence can support the diagnosis but is not mandatory.
Diagnostic Criteria for Post-Molar Trophoblastic Tumors
A diagnosis of gestational trophoblastic neoplasia (GTN) can be established during hCG follow-up after uterine evacuation of a molar pregnancy if any of the following criteria are met, provided residual pregnancy tissue or a new pregnancy is excluded:
- hCG levels plateau at a high level (±10%) over four measurements (days 1, 7, 14, and 21) for at least three consecutive weeks.
- hCG levels demonstrate a rise (>10%) on three consecutive measurements (days 1, 7, and 14) over at least two weeks.
- Histopathological confirmation indicates invasive mole or choriocarcinoma.
Diagnostic Criteria for Non-Post-Molar Trophoblastic Tumors
In cases of abnormal vaginal bleeding, or hemorrhage in the abdominal cavity, lungs, brain, or other organs following miscarriage, full-term delivery, or ectopic pregnancy, or in the presence of pulmonary or neurological symptoms, trophoblastic tumors should be considered. Serum hCG testing is essential. If abnormal hCG levels are detected, in conjunction with clinical presentation and after excluding residual pregnancy tissue or a new pregnancy, gestational trophoblastic tumor can be diagnosed.
Ultrasound Examination
This is the most common method for diagnosing primary uterine lesions. Ultrasonography may show a uterus of normal size or varying degrees of enlargement. High-echogenic masses with clear but unencapsulated borders may be visible in the myometrium. Alternatively, regions or masses of heterogeneous echogenicity with indistinct, unencapsulated borders may be observed. The uterus may also exhibit diffuse increased echogenicity with irregular hypo- or anechoic areas internally. Color Doppler ultrasound typically reveals abundant blood flow signals and low-resistance flow patterns.
Chest X-Ray
This routine examination identifies lung metastases. Typical findings include cotton-ball or mass-like opacities, with metastatic lesions more common in the right lung and mid-to-lower lung zones. Observable lesions on chest X-rays are included in the pulmonary metastasis count for prognostic scoring.
CT and MRI Imaging
Chest CT is capable of detecting smaller pulmonary lesions and is essential for diagnosing lung metastases. MRI is primarily used to identify brain, abdominal, and pelvic metastases. Chest CT is typically performed if chest X-rays are negative. Patients with positive findings on chest X-rays or CT should undergo additional CT or MRI imaging of the brain and liver.
Other Tests
Additional assessments include blood cell and platelet counts, as well as liver and kidney function tests.
Histopathological Diagnosis
The presence of chorionic villi or degenerated villous remnants in the uterine myometrium or extrauterine metastatic tissues indicates invasive mole. If histology reveals sheets of trophoblastic infiltration with necrosis and hemorrhage in the absence of villous structures, the diagnosis is choriocarcinoma. In cases where the primary and metastatic site diagnoses differ, the presence of villous structures in any tissue sample leads to a diagnosis of invasive mole. Although histopathological evidence is not essential for diagnosing gestational trophoblastic tumors, when available, histopathological findings take precedence in the diagnostic process.
Clinical Staging
The International Federation of Gynecology and Obstetrics (FIGO) staging system established by the Gynecological Oncology Committee is used for clinical staging. This system includes anatomical staging and a prognostic scoring system. Patients with a prognostic score of ≤6 are categorized as low risk, while those with a score of ≥7 are considered high risk. A prognostic score of ≥13, or the presence of extensive metastases to the liver or brain, and poor responsiveness to first-line combination chemotherapy indicates extremely high risk. For example, a patient with a trophoblastic tumor with lung metastases and a prognostic score of 6 would be staged as "Gestational Trophoblastic Tumor (III:6)."

Table 1 Anatomical staging of trophoblastic tumors (FIGO, 2000)
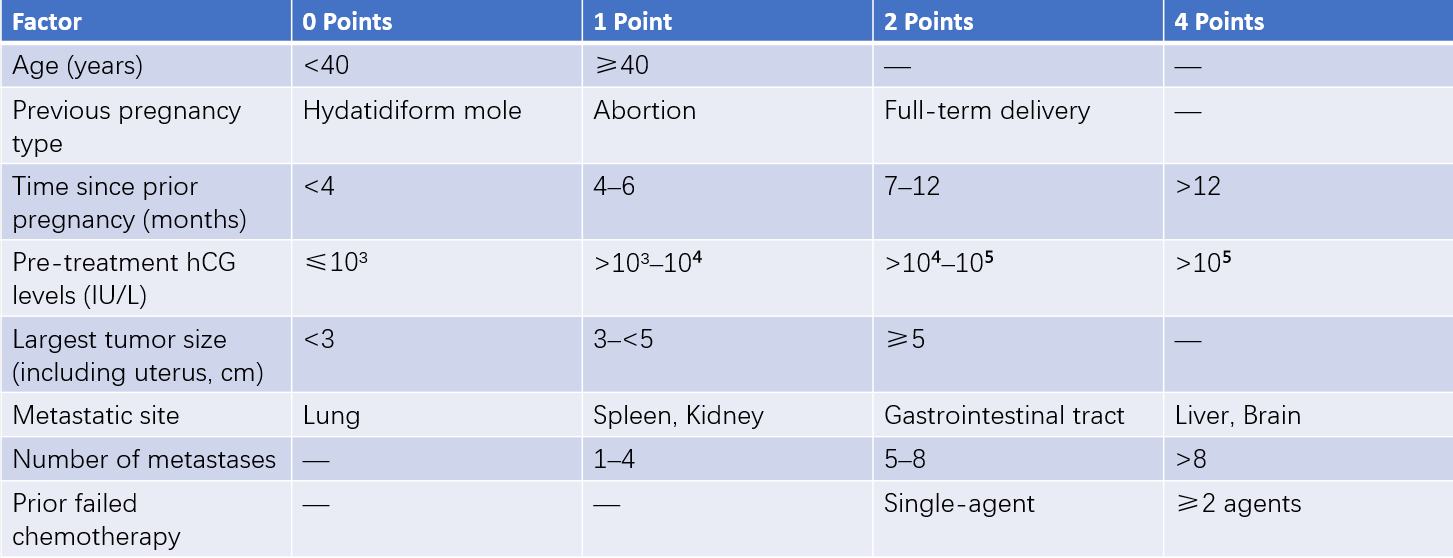
Table 2 FIGO/WHO prognostic scoring system for trophoblastic tumors (2000)
The prognostic score is a key factor in determining treatment protocols and evaluating prognosis for gestational trophoblastic tumors. Anatomical staging helps to clarify tumor progression and facilitates comparisons of treatment outcomes across medical institutions.
Treatment
The treatment principles prioritize chemotherapy as the primary modality, supplemented by surgical and radiation therapy. A definitive clinical diagnosis must first be established based on medical history, physical findings, and auxiliary test results. Accurate clinical staging is then undertaken, and patients are classified based on their prognostic scores as either low risk (typically including stage I–III with scores ≤6) or high risk (typically including stage I–III with scores ≥7 and stage IV). In conjunction with assessments of bone marrow function, liver and kidney function, and overall condition, appropriate treatment plans are designed based on a stratified approach.
Chemotherapy
Common first-line chemotherapeutic agents include methotrexate (MTX), actinomycin-D (Act-D), fluorouracil (5-FU)/fluorodeoxyuridine (FUDR), cyclophosphamide (CTX), vincristine (VCR), and etoposide (VP16). Monotherapy is often selected for low-risk patients. However, those with a prognostic score of 5–6 or pathological confirmation of choriocarcinoma face an increased risk of treatment failure with single-agent chemotherapy and may be treated with combination therapy following high-risk treatment protocols. High-risk patients typically receive combination therapy, with EMA-CO regimens or fluorouracil-based combinations as the first choice. For extremely high-risk patients, low-dose induction chemotherapy may precede second-line regimens such as EP-EMA. Extremely high-risk patients with bleeding risk or poor physical fitness may initially receive low-dose EP regimens for 2–3 cycles until stabilization, after which EP-EMA or other combination regimens are introduced.
- Single-Agent Chemotherapy: Commonly used single-agent chemotherapeutic drugs and their administration methods can be seen in Table 3.
- Combination Chemotherapy: EMA-CO regimens or fluorouracil-based combination regimens can be seen in Table 4.
- Efficacy Evaluation: Serum hCG levels should be measured weekly after the completion of each chemotherapy cycle and combined with gynecological examinations and imaging studies. Plateaued (changes <10%) or rising hCG levels after two consecutive cycles are considered treatment failures.
- Prevention of Toxic Side Effects: Common toxic side effects of chemotherapy include myelosuppression, followed by gastrointestinal reactions, liver and kidney damage, and alopecia. Baseline blood tests and liver/kidney function evaluations should be performed before initiating treatment. Patients need careful monitoring during chemotherapy to mitigate and manage any adverse effects.
- Criteria for Discontinuation: After hCG normalization, low-risk patients typically undergo 2–3 consolidation cycles, while high-risk patients undergo 3–4 consolidation cycles.
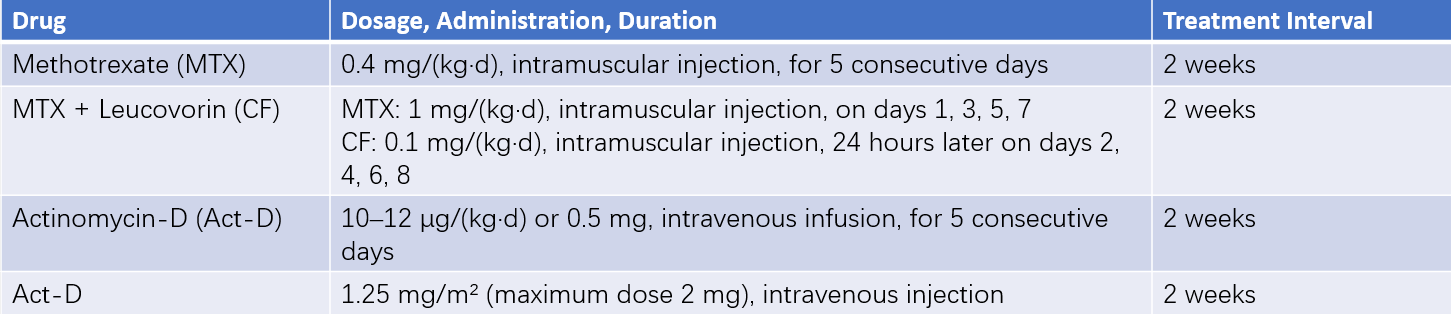
Table 3 Common single-agent chemotherapy regimens and administration
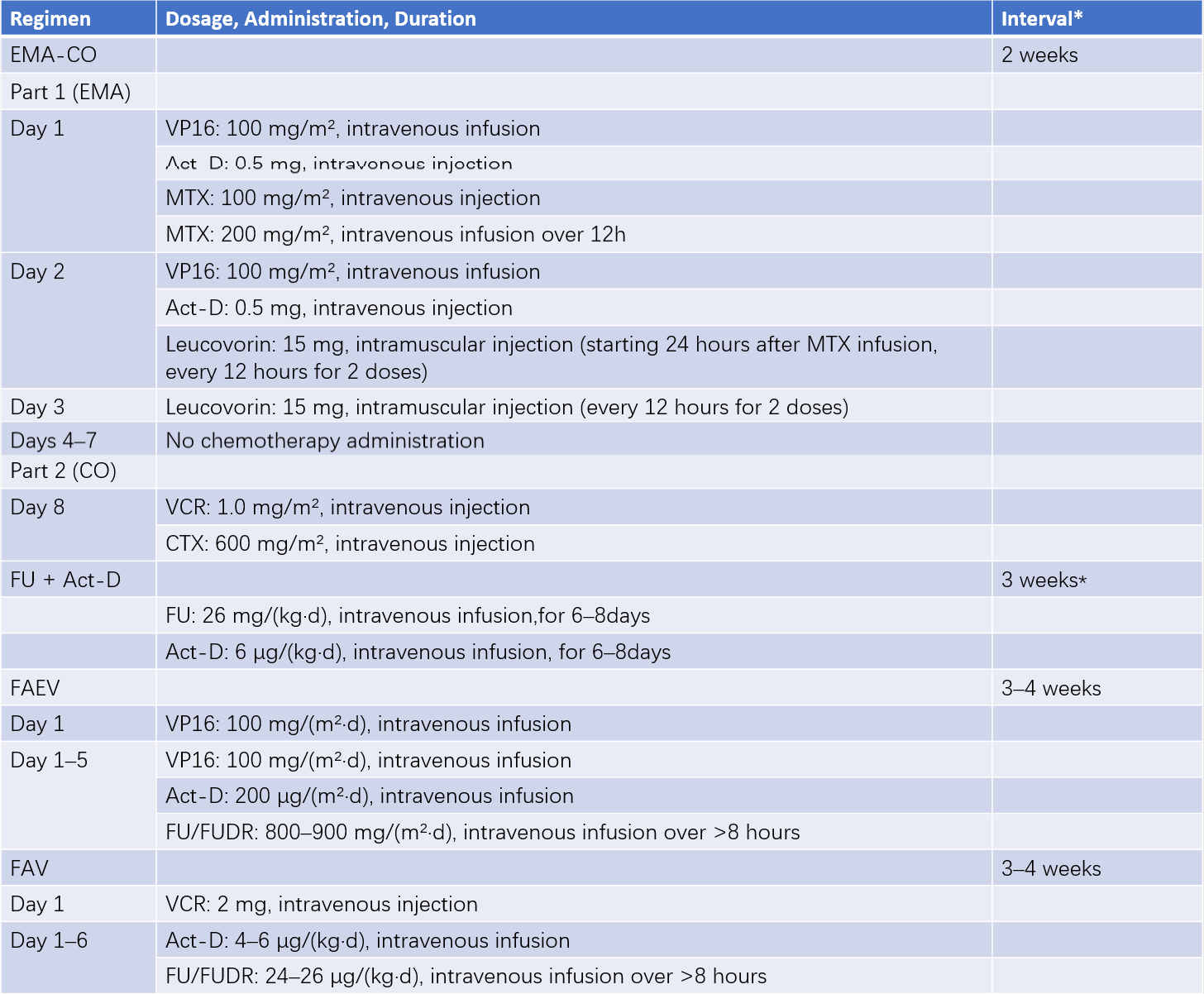
Table 4 Common combination chemotherapy regimens and administration
*Note: The interval refers to the time from the end of one chemotherapy cycle to the start of the next.
Surgical Treatment
Surgery serves as an adjunct to chemotherapy in specific situations. It is effective for controlling complications such as massive bleeding, removing isolated chemoresistant lesions, reducing tumor burden, and shortening chemotherapy duration. Surgical indications and timing for GTN include good general condition, localized and resectable lesions, absence of active lesions outside the surgical site, and chemoresistant disseminated lesions. Preoperative hCG levels should ideally be reduced to low levels, as surgery is contraindicated in the presence of rising hCG levels. Postoperative chemotherapy should be tailored with appropriate and sensitive regimens.
- Hysterectomy or Lesion Resection: Total hysterectomy is appropriate for patients without fertility requirements and no metastasis during initial treatment, with concurrent single-cycle adjunct chemotherapy during the surgery or multiple cycles until hCG normalizes. Patients with fertility requirements may undergo lesion resection and uterine repair if lesions have perforated and induced bleeding. Single uterine chemoresistant lesions with low hCG levels may also undergo lesion resection.
- Pulmonary Resection for Chemoresistant Lesions: Isolated pulmonary chemoresistant lesions with low hCG levels may be treated with wedge resection or lobectomy. Fibrotic nodules resulting from resorbed pulmonary metastases often persist visible on chest X-rays after hCG normalization, requiring careful differentiation before surgery.
Radiation Therapy
Radiation therapy is rarely used and is primarily focused on liver, brain metastases, or chemoresistant pulmonary lesions. Stereotactic brain radiotherapy, with or without intrathecal MTX injection, may be considered for certain brain metastases.
Treatment of Drug-Resistant or Recurrent Cases
Nearly all non-metastatic and low-risk metastatic patients achieve cure, but approximately 20% of high-risk metastatic cases result in drug resistance and recurrence, leading to eventual mortality. The management of such cases remains a significant challenge in the treatment of gestational trophoblastic neoplasia. Strategies include:
- Accurate staging and scoring before treatment to ensure standardized chemotherapy regimens and reduce resistance and recurrence risks.
- Combination chemotherapy regimens composed of effective second-line agents, including ifosfamide, platinum-based drugs, bleomycin, and paclitaxel. Regimens such as EP-EMA (EMA-CO with cisplatin and etoposide replacing Act-D and cyclophosphamide), PVB (cisplatin, vincristine, and bleomycin), BEP (bleomycin, etoposide, and cisplatin), VIP (etoposide, ifosfamide, and cisplatin or carboplatin), and TP/TE (paclitaxel plus cisplatin or etoposide) are commonly used.
- Emerging research suggests that the combination of PD-1/PD-L1 monoclonal antibodies with chemotherapy or anti-angiogenic targeted therapies is an effective option for patients with recurrent, drug-resistant high-risk GTN.
Follow-Up
Close surveillance is required after the completion of treatment. hCG levels should be monitored monthly for the first year, every three months during the second and third years, and annually during the fourth and fifth years. The follow-up protocol is similar to that for molar pregnancies. Strict contraception should be practiced throughout the follow-up period for at least one year, and pregnancy is generally advised no earlier than 12 months after cessation of chemotherapy.