A hydatidiform mole forms due to trophoblastic hyperplasia and stromal edema of placental villi after conception, resulting in the formation of variably sized vesicles. These vesicles are interconnected by pedicles, resembling a cluster of grapes. It is also referred to as a molar pregnancy. Hydatidiform moles are categorized into two types: complete hydatidiform mole and partial hydatidiform mole.
Associated Factors
Complete Hydatidiform Mole
The incidence of hydatidiform mole is approximately 2 per 1,000 pregnancies in some parts of Asia, whereas it is less than 1 per 1,000 pregnancies in Europe and North America. In recent years, the incidence of hydatidiform mole has decreased in some Asian countries, likely due to factors such as economic development, improved diets, and declining fertility rates. Globally, the incidence is approximately 0.81‰ (per 1,000 pregnancies), or 1 in 1,238 pregnancies if calculated per multiple pregnancies instead of rates. Among populations of the same ethnicity, the incidence of hydatidiform mole can vary regionally. For example, the incidence among Jewish descendants in North Africa and Eastern countries is twice as high as that in Western countries, suggesting that factors beyond ethnicity, such as environmental or socioeconomic conditions, contribute to regional differences.
Nutritional status and socioeconomic factors are potential risk factors. A diet deficient in vitamin A, β-carotene, and animal fats significantly increases the likelihood of developing hydatidiform mole. Age is another related factor, with women over 35 and over 40 years old experiencing rates that are 2 times and 7.5 times higher, respectively, than those of younger women. Pregnancy in women over 50 years of age carries a one-third probability of resulting in a hydatidiform mole. Conversely, the condition is also more common in women under 20 years of age. A history of prior molar pregnancy is another high-risk factor, with recurrence rates of 1% after one molar pregnancy and 15%-20% after two. Additionally, a history of miscarriage or infertility may pose elevated risks.
The chromosomal karyotype of complete hydatidiform moles is diploid, and all chromosomes are of paternal origin. About 90% of cases exhibit a 46,XX karyotype, which results from fertilization of an enucleated or inactive egg by a single haploid sperm (23,X), followed by its duplication to form a diploid genome (46,XX). The remaining 10% of cases involve a 46,XY karyotype, resulting from fertilization of an enucleated egg by two haploid sperm (23,X and 23,Y). Although complete hydatidiform moles are genetically paternal in origin, their mitochondrial DNA is derived maternally.
Partial Hydatidiform Mole
Partial hydatidiform mole has been considered less common than complete mole. However, recent studies indicate that the proportion of partial to complete hydatidiform moles is now approximately equal or even higher, with rates reported at 0.78 and 1.13 by studies in Japan and the UK, respectively. The observed increase may be due to declining rates of complete molar pregnancy and improvements in the diagnostic precision of partial hydatidiform mole cases. Many early miscarriages with triploidy are now definitively identified as partial hydatidiform moles through pathological and molecular genetic analysis.
Risk factors for partial hydatidiform mole remain poorly understood; possible associations include irregular menstruation and oral contraception, but maternal age and diet appear unrelated.
More than 90% of partial hydatidiform moles exhibit a triploid karyotype, with the associated fetus also being triploid. The most common karyotype is 69,XXY, with others including 69,XXX and 69,XYY. These result from fertilization of a seemingly normal haploid egg by two haploid sperm or by a single diploid sperm with defective meiosis. The additional chromosome is paternal in origin, and the extra paternal genetic material is the primary cause of trophoblastic hyperplasia in partial hydatidiform moles. Rare cases of tetraploid partial moles have also been reported, though the mechanism of their formation is unclear.
Pathology
Complete Hydatidiform Mole
The vesicular structures vary in size, with diameters ranging from millimeters to several centimeters. They are connected by delicate fibrous tissue and are often accompanied by blood clots and fragments of decidual tissue. The vesicles fill the entire uterine cavity, with the absence of fetal tissues or associated structures.
Microscopic findings include:
- Absence of embryonic or fetal tissue.
- Profound edema of the villi, often forming central fluid-filled spaces (cisterns), with the disappearance of vascular structures.
- Diffuse trophoblastic proliferation with marked atypia.
- Almost always accompanied by exaggerated placental site reaction.
Complete hydatidiform moles lack maternal chromosomes and do not express imprinted paternal or maternally expressed genes such as P57KIP2. Accordingly, immunohistochemistry for P57 is negative.
Partial Hydatidiform Mole
Only part of the placental villi is hydropic. Embryonic or gestational sac structures may be identifiable, although the fetus is often deceased and generally exhibits growth retardation or multiple congenital anomalies; term fetuses are exceedingly rare.
Microscopic findings include:
- Presence of embryonic or gestational sac components.
- Coexistence of hydropic villi and morphologically normal villi.
- Localized, mild trophoblastic hyperplasia.
- Scalloped contours of the villi with trophoblastic inclusions within the stroma.
Unlike complete moles, partial hydatidiform moles have a biparental chromosomal origin, allowing for expression of paternally imprinted and maternally expressed genes. Immunohistochemistry for P57 is consequently positive.
Clinical Manifestations
Complete Hydatidiform Mole
With advances in diagnostic techniques, many cases of hydatidiform mole are now diagnosed and managed in early pregnancy, making the presentation of classic clinical features increasingly rare. The typical clinical manifestations of complete hydatidiform mole include:
Postmenopausal Vaginal Bleeding
This is the most common symptom. Irregular vaginal bleeding usually begins 8 to 12 weeks after amenorrhea. Due to the invasive nature of trophoblastic cells into blood vessels, this may lead to significant hemorrhage, shock, and, in some cases, death. Hydatidiform mole tissue may sometimes be spontaneously expelled, often accompanied by heavy bleeding both before and during expulsion. Recurrent vaginal bleeding, if untreated, can result in secondary anemia and infection.
Abnormal Uterine Enlargement and Softening
Rapid growth of the molar tissue, along with intrauterine blood accumulation, causes the uterus to appear larger than expected for the duration of amenorrhea and to have a softened consistency. However, in some cases, the uterine size may correspond to or be smaller than the gestational age, which may be related to degenerative changes in the vesicles.
Pregnancy-Associated Vomiting
Vomiting is common in cases of uterine enlargement and abnormally elevated hCG levels. Symptoms often begin earlier, are more severe, and persist longer than in normal pregnancies. Severe, uncorrected vomiting may result in electrolyte imbalances.
Signs of Preeclampsia
These are more likely to occur in cases of significantly enlarged uterus and may present as hypertension, proteinuria, and edema prior to 24 weeks of gestation, though eclampsia is rare.
Hyperthyroidism
Symptoms include tachycardia, moist skin, and tremors, with elevated serum free T3 and T4 levels. However, exophthalmos is rare.
Abdominal Pain
This is typically related to the rapid growth of the hydatidiform mole and the rapid expansion of the uterus, presenting as intermittent lower abdominal pain. It is generally mild and tolerable, often preceding vaginal bleeding. Acute abdominal pain may occur if an ovarian theca-lutein cyst ruptures or undergoes torsion.
Ovarian Theca-Lutein Cysts
These are caused by high levels of hCG stimulating luteinization of follicular theca cells. About half of patients with complete hydatidiform mole develop notable theca-lutein cysts, which are typically bilateral, though they may occur unilaterally, and vary in size. They are generally asymptomatic. Due to uterine overgrowth, the cysts are often not palpable during gynecological examinations and are usually diagnosed by ultrasonography. These ovarian cysts typically resolve spontaneously within 2 to 4 months after evacuation of the mole.
Partial Hydatidiform Mole
Similar to complete mole, partial hydatidiform mole often presents with vaginal bleeding after amenorrhea. The clinical presentation may resemble that of incomplete or missed abortion. Other symptoms are less pronounced and milder compared to complete hydatidiform mole.
Natural Course
Under normal circumstances, serum hCG levels progressively decline after evacuation of the hydatidiform mole. The average time required for hCG levels to return to normal is approximately 9 weeks, with a maximum duration of 14 weeks. Persistent abnormal hCG levels following evacuation of the mole should raise suspicion for gestational trophoblastic neoplasia.
The likelihood of uterine invasion or distant metastasis is approximately 15% and 4%, respectively, in cases of complete hydatidiform mole. Cases exhibiting one or more of the following risk factors are classified as high-risk:
- hCG levels >100,000 IU/L,
- Uterine size significantly larger than gestational age,
- Theca-lutein cysts >6 cm in diameter.
Additionally, advanced maternal age (>40 years) and a history of recurrent hydatidiform mole are also considered high-risk factors.
Partial hydatidiform mole has a lower likelihood of uterine invasion, with an incidence of uterine invasion ranging from 1% to 4%. Distant metastasis is rare. Unlike complete mole, partial mole lacks prominent clinical or pathological high-risk factors.
Diagnosis
Hydatidiform mole should be considered in cases of irregular vaginal bleeding after amenorrhea, abnormal uterine enlargement, elevated hCG levels, and/or signs of preeclampsia in early pregnancy. The diagnosis is supported if grape-like vesicular tissues are expelled vaginally or if ultrasonography shows a "honeycomb" or "snowstorm" pattern. Histological confirmation remains the definitive basis for diagnosis. When hydatidiform mole is suspected clinically, the following auxiliary tests are often employed to confirm the diagnosis:
Ultrasonography
Ultrasonography is a commonly used diagnostic method, with transvaginal color Doppler ultrasonography being the preferred approach. For complete hydatidiform mole, typical ultrasound findings include: a uterus larger than expected for gestational age, absence of a gestational sac or fetal heartbeat, and a uterine cavity filled with heterogeneous echogenic masses resembling a "snowstorm" pattern. When the vesicles are sufficiently large, they appear as a "honeycomb" pattern. Unilateral or bilateral ovarian cysts are often observed. Color Doppler imaging tends to reveal rich blood flow in the uterine arteries, while blood flow within the myometrium is sparse or absent. For partial hydatidiform mole, focal vesicular changes in the placenta can create characteristic ultrasound images. In some cases, a fetus or amniotic sac may be visible, though the fetus is usually malformed. Early-stage hydatidiform moles can display atypical ultrasound features, increasing the likelihood of misdiagnosis.
Quantitative Measurement of hCG
Serum hCG measurement is another important diagnostic method. In normal pregnancies, trophoblastic cells begin secreting hCG a few days after implantation, with serum hCG levels rising progressively, peaking at 8–10 weeks of gestation, and then gradually declining over 1–2 weeks. In cases of hydatidiform mole, serum hCG levels are often significantly higher than those expected for the gestational age and may continue to rise beyond 8–10 weeks of amenorrhea. Approximately 45% of patients with complete hydatidiform mole have serum hCG levels exceeding 100,000 IU/L, with some reaching as high as 2.4 million IU/L. However, a small subset of moles, particularly partial hydatidiform moles with degenerative changes in the villi, show less pronounced hCG elevation.
DNA Ploidy Analysis
DNA ploidy analysis, commonly performed using flow cytometry, is a widely used tool. Complete hydatidiform moles exhibit a diploid karyotype, while partial hydatidiform moles exhibit a triploid karyotype.
Imprinted Gene Testing
Partial hydatidiform moles possess biparental chromosomes and therefore express both paternally imprinted and maternally expressed genes (e.g., P57KIP2). In contrast, complete hydatidiform moles lack maternal chromosomes and thus do not express such genes. Immunohistochemical staining for P57 can distinguish between complete and partial hydatidiform moles.
Molecular Genotyping
Short tandem repeats (STRs), which are highly polymorphic, genetically stable segments of short repeating DNA sequences in non-coding regions of the human genome, are used for molecular analysis. STR comparison between pregnancy and maternal tissues can determine the chromosomal origin of the embryo, facilitating precise diagnosis and risk stratification of hydatidiform mole.
Other Tests
Additional tests, such as chest X-rays, blood cell and platelet counts, liver and kidney function tests, thyroid function tests, and blood typing, are often performed.
Differential Diagnosis
Complete vs Partial Hydatidiform Mole
Early diagnosis of most complete hydatidiform moles can limit the appearance of classic clinical features, leading to misdiagnosis as partial hydatidiform mole. Differentiation is crucial due to differing clinical outcomes. Identification relies primarily on gross and histopathological features, P57 staining, DNA ploidy analysis, and STR testing. A summary of key distinctions in karyotype and pathological features can clarify the differences.
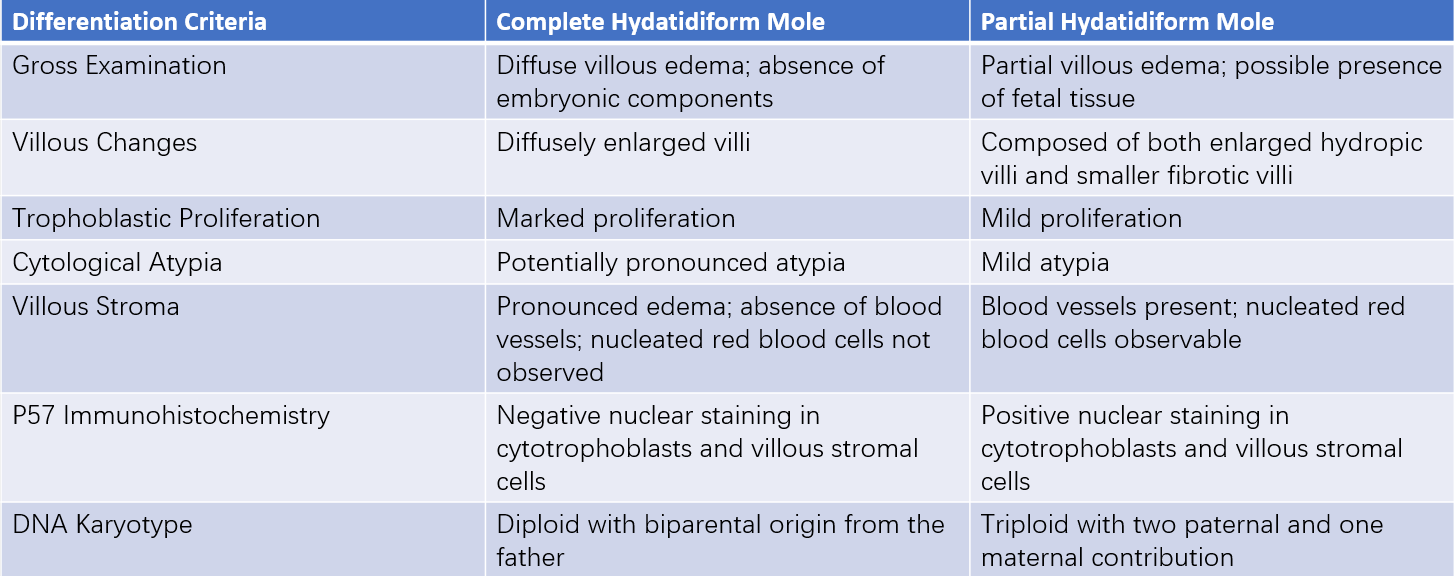
Table 1 Distinction between complete hydatidiform mole and partial hydatidiform mole
Miscarriage
The clinical history of hydatidiform mole may resemble that of miscarriage, leading to potential misdiagnosis. Differentiating partial hydatidiform mole from miscarriage can be particularly challenging, even histopathologically, as villous edema and minimal trophoblastic proliferation may obscure findings. DNA ploidy analysis and STR testing are often necessary for differentiation.
Cesarean Scar Pregnancy
Cesarean scar pregnancy is a complication following cesarean delivery, where the gestational sac implants at the site of a uterine incision scar. It presents with vaginal bleeding after amenorrhea, mimicking hydatidiform mole. Ultrasonography can assist in differentiation.
Twin Pregnancy
Twin pregnancy can lead to uterine enlargement greater than expected for gestational age and slightly elevated hCG levels, resembling hydatidiform mole. However, vaginal bleeding is not typically observed in twin pregnancies, and ultrasonography can confirm the diagnosis.
Treatment
Uterine Evacuation
Once the diagnosis of hydatidiform mole is confirmed, uterine evacuation should be performed promptly. Prior to the procedure, it is important to assess for complications such as shock, preeclampsia, hyperthyroidism, or anemia, and provide symptomatic treatment to stabilize the patient's condition if necessary. The procedure should be conducted under ultrasound guidance by an experienced gynecologist. Suction curettage is the commonly employed method due to its advantages of shorter operative time, reduced bleeding, and lower risk of uterine perforation. Hydatidiform mole removal often involves significant bleeding, and the uterus may be large and soft, which increases the risk of perforation. For this reason, the procedure is conducted in an operating room setting with intravenous fluid administration and blood products readily available. The cervical canal is dilated, and a large suction catheter is used for evacuation. Once most of the molar tissue is removed and the uterus has visibly shrunk, gentle curettage with a curette is performed. To reduce bleeding and prevent uterine perforation, oxytocin may be administered intravenously after adequate cervical dilation and the initiation of suction evacuation. Oxytocin use does not typically increase the risk of trophoblastic metastasis or pulmonary embolism. A single evacuation is usually sufficient, but a second procedure may be required in cases of persistent uterine bleeding or ultrasound findings indicating retained products of conception.
During the procedure, trophoblastic embolism of uterine venous sinuses may lead to pulmonary artery embolism, acute respiratory distress, or acute right heart failure. Such events require timely management with supportive therapies for cardiovascular and respiratory function, and recovery typically occurs within 72 hours. Acute respiratory distress may also occur due to complications such as hyperthyroidism or preeclampsia. For safety, patients with uterine size equivalent to a gestation beyond 16 weeks or with significant complications should undergo the procedure in specialized healthcare facilities with relevant expertise.
All curettage specimens from the evacuation must undergo histological examination. Tissue sampling should focus on fresh, non-necrotic material from near the implantation site.
Management of Ovarian Theca-Lutein Cysts
Theca-lutein cysts generally resolve spontaneously following uterine evacuation and do not usually require intervention. In cases of acute torsion, aspiration under ultrasound guidance or laparoscopic puncture may facilitate spontaneous resolution of the torsion. If prolonged torsion results in necrosis, ipsilateral adnexectomy may become necessary.
Prophylactic Chemotherapy
Although evidence suggests that prophylactic chemotherapy during evacuation may reduce the probability of gestational trophoblastic neoplasia (GTN) in high-risk molar pregnancies, it is not routinely recommended. Prophylactic chemotherapy may be considered in patients with complete hydatidiform mole who have high-risk factors and/or for whom follow-up is challenging, but it remains a non-standard approach. If administered, prophylactic chemotherapy should be carried out before or during evacuation using a single-agent regimen and continuing for multiple cycles until hCG levels normalize. Prophylactic chemotherapy is not performed for partial hydatidiform mole.
Hysterectomy
Patients with no desire for future childbearing may consider total hysterectomy with bilateral salpingectomy. Hysterectomy does not prevent extrauterine metastasis of hydatidiform mole and is therefore not routinely recommended, except in cases where other indications necessitate uterine removal. Postoperative follow-up remains necessary.
Follow-Up
Regular follow-up is essential for patients after uterine evacuation of hydatidiform mole to detect and address trophoblastic neoplasia as early as possible. Follow-up involves the following components:
- hCG Monitoring: Serum hCG is measured weekly after treatment until three consecutive results are negative, followed by monthly measurements for six months.
- Symptom Inquiry: The clinical history is reviewed to assess menstrual cycles and check for symptoms such as vaginal bleeding, cough, or hemoptysis.
- Gynecological Examination: Additional investigations, such as ultrasonography, chest X-rays, or CT scans, may be conducted as needed.
Reliable contraception is necessary during the follow-up period for molar pregnancy. As most cases of trophoblastic neoplasia occur before hCG levels normalize, the recommended contraception duration is six months. If conception occurs before the conclusion of the six-month follow-up but hCG levels had already normalized prior to conception, the pregnancy does not need to be terminated. However, early ultrasonography and hCG measurement are advisable to confirm the viability of the pregnancy, and postpartum hCG monitoring should continue until normalization is confirmed. Contraceptive options may include oral contraceptive pills or condoms. Intrauterine devices are not preferred to avoid confusion with uterine bleeding causes or the risk of perforation.