Cervical squamous intraepithelial lesion (SIL) refers to a group of cervical abnormalities closely associated with invasive cervical carcinoma. This category includes histologically confirmed squamous intraepithelial lesions and glandular intraepithelial lesions, which are regarded as precursor lesions of cervical cancer.
Associated Risk Factors
Persistent infection with high-risk human papillomavirus (HPV) serves as the most important causative factor for cervical cancer and its precursor lesions. High-risk HPV has been identified in nearly 90% of cervical squamous intraepithelial lesions and cervical cancer. HPV infections are often transient, asymptomatic, and most are cleared within two years. However, fewer than 5% of persistent infections progress to precursor lesions and invasive carcinoma. HPV is an epitheliotropic, small, double-stranded circular DNA virus lacking an envelope. Over 200 HPV genotypes have been identified, of which more than 40 are associated with anogenital infections. Based on biological characteristics and oncogenic potential, HPV genotypes are categorized into high-risk and low-risk types. The 14 high-risk types include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, which are strongly associated with cervical cancer and its precursor lesions. Low-risk types, such as HPV6, 11, 42, 43, and 44, are mainly associated with genital warts and other benign lesions. Among these, HPV16 has the highest oncogenic potential, with approximately 70% of cervical cancers linked to persistent infections with HPV16 and 18. Following integration of high-risk HPV DNA into the host cervical epithelial cell genome, the viral E6 and E7 oncoproteins are persistently expressed, targeting and inactivating the host cell's p53 and Rb proteins. These molecular events eventually lead to cervical intraepithelial lesions and malignant transformation. Other risk factors include multiple sexual partners, early sexual activity (before 16 years), high parity, sexually transmitted diseases, immune suppression or dysfunction, smoking, oral contraceptive use, and poor nutrition.
Epidemiology of HPV Infection
Approximately 80% of sexually active adult women acquire one or more HPV subtypes at some point in their lives. The infection rate and distribution of subtypes vary across different regions and countries. In high-incidence regions for cervical cancer, the infection rate is 10%–20% or higher, whereas in low-incidence regions, the rate is around 5%–10%.
Histological Characteristics of Cervical Tissue
The cervix is composed of the squamous epithelium of the ectocervix and the columnar epithelium of the endocervical canal.
Squamous Epithelium of the Ectocervix
The ectocervical squamous epithelium consists of four layers: the basal layer, the parabasal layer, the intermediate layer, and the superficial layer. The basal and parabasal layers consist of basal cells and parabasal cells, forming the germinal layer responsible for epithelial regeneration. Basal cells act as stem cells and show minimal proliferative activity under normal conditions but may proliferate abnormally or differentiate into mature squamous cells under stimuli. Parabasal cells are highly proliferative, often exhibiting mitotic activity and expressing Ki67. Intermediate and superficial layers contain terminally differentiated cells that undergo gradual cell death and desquamation.
Columnar Epithelium of the Endocervical Canal
The columnar epithelium lines the surface and glands of the cervical canal. These cells have basal nuclei and mucus-filled cytoplasm. Reserve cells, located beneath the columnar epithelium, possess the ability to either differentiate or proliferate.
Cervical Transformation Zone
The transformation zone, also known as the squamo-columnar junction (SCJ), marks the interface between the squamous epithelium and the columnar epithelium. This junction shifts dynamically with age and physiological changes, resulting in its classification into the original squamo-columnar junction and the physiological squamo-columnar junction.
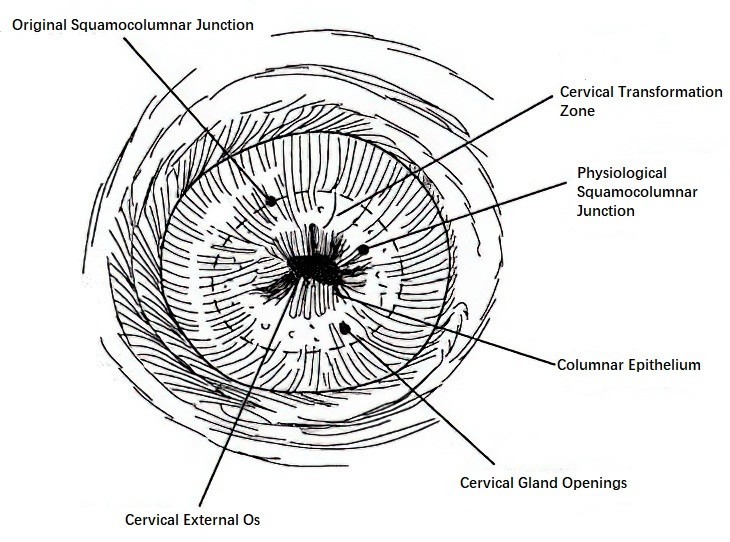
Figure 1 Cervical transformation zone and squamocolumnar junction
During fetal development, squamous epithelium derived from the urogenital sinus grows toward the cervix, forming the original SCJ at the junction with the columnar epithelium of the cervical canal. After puberty and under the influence of estrogen, the cervix enlarges, and the cervical canal’s mucosal tissue extends caudally. As a result, columnar epithelium from the cervical canal and its underlying stroma are exposed on the ectocervix, shifting the original SCJ outward. On the inner side of the original SCJ, the columnar epithelium is thin, and its underlying stroma gives it a red, granular appearance called columnar ectopy or "erosion-like changes" when viewed macroscopically. Over time, under the acidic environment of the vagina or pathogen exposure, the exposed columnar epithelium is gradually replaced by squamous epithelium, forming the new physiological SCJ.
The area between the original SCJ and the new physiological SCJ is referred to as the transformation zone. During its formation, newly developed squamous epithelium may overgrow or infiltrate gland openings, occluding them with connective tissue proliferation or scarring. This process can cause glandular secretions to accumulate, forming cervix retention cysts, known as Naboth cysts, which serve as markers of the transformation zone. Postmenopause, estrogen levels decline, the cervix atrophies, and the original SCJ recedes into the cervical canal.
Mechanisms for the replacement of columnar epithelium in the transformation zone include:
- Squamous Metaplasia: Columnar epithelium exposed on the ectocervix transforms into squamous epithelium under the influence of the vaginal acidic environment, with reserve cells beneath the columnar epithelium proliferating and differentiating into squamous epithelium. The replaced columnar epithelium then sloughs off.
- Squamous Epithelization: Squamous epithelium from the ectocervix directly grows into the interface between the columnar epithelium and its basement membrane, replacing the entire columnar structure.
Mature squamous epithelium in the transformation zone is relatively resistant to carcinogenic agents, while immature squamous metaplastic epithelium, due to its metabolic activity, is prone to abnormalities under factors such as HPV infection. These abnormalities include dysregulated cell proliferation, differentiation, disordered cellular arrangement, nuclear abnormalities, and increased mitotic activity, ultimately resulting in cervical squamous intraepithelial lesions. Consequently, the transformation zone serves as the most common site for cervical cancer development. Additionally, glandular intraepithelial lesions may occur in the columnar epithelium of the cervical canal.
Pathological Diagnosis and Grading
Cervical intraepithelial lesions are histologically classified into squamous intraepithelial lesions and glandular intraepithelial lesions.
Squamous Intraepithelial Lesion (SIL)
Squamous intraepithelial lesion (SIL), formerly termed cervical intraepithelial neoplasia (CIN), is graded into three levels. Currently, a two-tier classification system aligned with cytological nomenclature is adopted. Low-grade squamous intraepithelial lesion (LSIL) corresponds to CIN1, while high-grade squamous intraepithelial lesion (HSIL) encompasses CIN2 and CIN3. When it is difficult to distinguish between LSIL and HSIL in CIN2 based on histology, p16 immunohistochemical staining may assist in diagnosis, where p16-negative cases are categorized as LSIL and p16-positive cases as HSIL. The two-tier classification offers simplicity and practicality, improves diagnostic reproducibility, better reflects the biological behavior of HPV-associated lesions, and facilitates clinical treatment and prognostic assessment. According to the 2020 WHO Classification of Tumors of Female Reproductive Organs, CIN2 and CIN3 should be specified in HSIL diagnoses to enable more precise clinical management. CIN3 demonstrates greater diagnostic reproducibility and an HPV genotype distribution closer to invasive cancer; thus, it is considered the primary clinical endpoint for assessing cervical cancer risk.
Most HSIL cases are associated with persistent HPV infection and represent precursor lesions for squamous cell carcinoma of the cervix. Without treatment, there is a risk of progression to invasive carcinoma.
LSIL
In LSIL, there is hyperplasia of the basal and parabasal cells, mild nuclear polarity disruption, and slight atypia, with limited mitotic activity, confined to the lower one-third of the epithelium. The upper two-thirds of the epithelium consist of differentiated, mature epithelial cells, often with atypical koilocytes, which are a characteristic manifestation of HPV infection showing nuclear enlargement with a perinuclear halo. P16 immunohistochemistry may assist in diagnosis.

Figure 2 Low-grade squamous intraepithelial lesion
HSIL
In HSIL, there is full-thickness nuclear atypia across the squamous epithelium, with hyperchromatic nuclei, coarse chromatin, irregular nuclear membranes, increased nuclear-cytoplasmic ratio, and frequent mitotic figures.
- CIN2 lesions exhibit dysplastic squamous epithelium extending to the middle one-third of the epithelium, with evidence of maturation in the upper one-third layer.
- CIN3 lesions show dysplastic basal/parabasal cell hyperplasia involving more than two-thirds of the epithelial thickness, reaching the upper one-third layer without associated maturation. CIN2 and CIN3 lesions can display atypical mitotic figures throughout the full epithelial thickness, including the superficial layer. Immunohistochemical staining for p16 typically shows intense and diffuse block-like staining.

Figure 3 High-grade squamous intraepithelial lesion
Glandular Intraepithelial Lesion
Glandular intraepithelial lesions, previously termed adenocarcinoma in situ (AIS), are also referred to as high-grade glandular intraepithelial lesions. These are high-grade epithelial abnormalities of glandular origin and serve as precursor lesions for cervical adenocarcinoma. If untreated, there is a risk of progression to invasive adenocarcinoma. Some AIS cases are not associated with high-risk HPV infection. The 2020 WHO Classification of Tumors of Female Reproductive Organs further divides AIS into HPV-associated AIS and non-HPV-associated AIS.
AIS
The lesion primarily occurs in the cervical canal, involving the surface epithelium and glands while maintaining a normal lobular architecture.
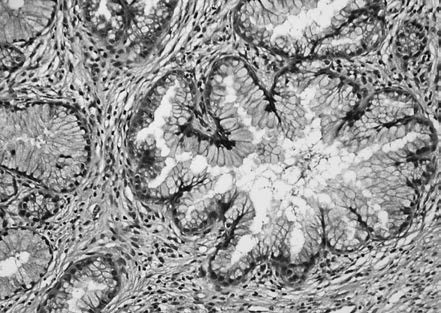
Figure 4 HPV-associated adenocarcinoma in situ (AIS)
HPV-associated AIS lesions show columnar epithelium covering the mucosa or glands, with reduced mucin in the cytoplasm. The epithelial cells are often pseudostratified, with enlarged, hyperchromatic nuclei, frequent mitoses near the luminal border, and basal apoptotic bodies. A specific subtype of HPV-associated AIS termed "stratified mucin-producing intraepithelial lesion" (SMILE) consists of stratified epithelium with intracellular mucin but lacks well-formed glands. Immunohistochemistry for p16 typically shows diffuse, strong positivity, with high Ki67 proliferation index and negative expression for ER and PR.
Gastric-type AIS, a non-HPV-associated subtype, features cuboidal or columnar tumor cells with clear boundaries, eosinophilic or clear cytoplasm, and minimal atypia in well-differentiated cases, while poorly differentiated lesions show significant nuclear atypia. Atypical lobular endocervical glandular hyperplasia (ALEGH) with gastric differentiation can be regarded as a precursor lesion for gastric-type adenocarcinoma. Non-HPV-associated AIS typically shows negative or patchy expression for p16 and abnormal p53 immunostaining, supporting the diagnosis.
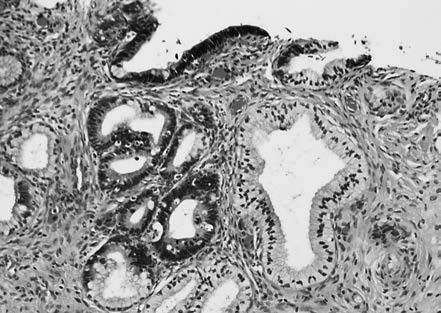
Figure 5 Atypical lobular cervical glandular hyperplasia
Outcomes
Three possible outcomes for cervical intraepithelial lesions include regression, persistence, and progression. CIN1 exhibits a high regression rate, with most cases resolving spontaneously and only a small proportion persisting. Untreated, approximately 10% of CIN1 cases progress to CIN3. CIN2 regressions occur in 50% of cases, with only 18% progressing. Young women exhibit higher regression rates and lower progression rates. CIN3 and AIS carry a high risk of progression to invasive carcinoma if left untreated.
Clinical Features
Cervical intraepithelial lesions do not typically present with specific symptoms. Occasionally, there may be increased vaginal discharge, with or without odor. Postcoital bleeding or contact bleeding after gynecological examination may also be observed. The cervix generally appears smooth, with some cases showing localized erythema, white epithelium, or erosion-like changes without obvious visible lesions.
Diagnosis
The diagnostic process follows a three-step approach, including cervical cancer screening, colposcopy referrals for abnormal screening results, and histopathological diagnosis.
Screening
Cervical cancer screening is an effective method for identifying cervical intraepithelial lesions and early-stage cervical invasive carcinoma in sexually active, age-appropriate women.
Screening Methods
HPV Nucleic Acid Testing
This approach specifically detects nucleic acids for 14 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The test can either be non-genotyping or partially genotyping for types 16 and 18. HPV testing serves as the primary cervical cancer screening method in healthy women aged 25 years and older. It demonstrates high sensitivity for detecting CIN2 and more severe lesions, identifying abnormalities 3–5 years earlier than cytological methods, although its specificity is lower. For this reason, HPV-positive results require triage to avoid overdiagnosis and overtreatment. Triage methods may include cytology or partial genotyping for HPV16/18.
HPV testing can also be used to triage cases with mildly abnormal cytology, to manage cervical lesions, and for post-treatment follow-up surveillance. The HPV detection technique and reagents should be validated and approved by authoritative institutions domestically or internationally to ensure their suitability for primary screening.
Cervical Cytology Examination
This is one of the earliest methods used for cervical cancer screening. Cytology methods include the Pap smear and liquid-based cytology. The Bethesda System (TBS) is commonly used for reporting cytology results, as it effectively integrates cytological, histological, and clinical management strategies. Cytology demonstrates high specificity (>90%), allowing immediate risk assessment, but has lower sensitivity for diagnosing CIN2, CIN3 (47%–62%), and AIS (approximately 50%), which may lead to missed high-grade lesions. Cytology is not a preferred primary screening option for women aged 25 years or older but can be used for those under 25. It can also be performed alone or combined with HPV testing for screening women aged 25 years or older and for triage in HPV-positive individuals.
Co-Testing
Co-testing combines HPV nucleic acid testing with cytological examination. This combined method takes advantage of the strengths of both and is suitable for cervical cancer screening in regions with abundant medical resources.
Visual Inspection
This approach involves visual inspection with acetic acid or iodine staining. After applying acetic acid or an iodine solution, the cervical surface is visually examined for discoloration or abnormal areas. This method has limited sensitivity and specificity, and it is less commonly used, typically restricted to regions with limited healthcare resources.
Other Screening Methods
Emerging methods include methylation assays, HPV integration testing, HPV DNA load testing, immunocytochemical staining, and artificial intelligence applications. These approaches show promise in screening but require further validation in large-scale prospective studies.
Screening Types
Population-Based Screening
Organized and systematic screening is carried out among age-appropriate women with a history of sexual activity.
Opportunistic Screening
Screening is conducted for age-appropriate women with a history of sexual activity who are visiting medical institutions for other reasons.
Screening Protocols
Screening Initiation Age
Screening begins at age 25. The incidence of cervical cancer in women under 25 is less than 1%. Intervening too early may adversely affect future pregnancy outcomes.
Screening for Women Aged 25–64
Screening options include HPV nucleic acid testing every five years, co-testing every five years, or cytological examination every three years.
Screening Termination Age
Screening may be discontinued at age 65 if, within the past 10 years, there have been two consecutive normal HPV or co-testing results or three consecutive normal cytology results, with the most recent screening conducted within the last five years. Women must also be free of any history of HPV-related disease or other high-risk factors for cervical cancer.
Women with high-risk factors for cervical cancer may initiate screening before age 25, with recommendations to start within one year of initiating sexual activity and to shorten the screening intervals accordingly.
Colposcopy
Colposcopy is a critical step for diagnosing cervical intraepithelial lesions and early cervical cancer. It identifies the lesion site and guides biopsy and treatment. Abnormal screening results requiring referral for colposcopy include HPV positivity coupled with atypical squamous cells of undetermined significance (ASC-US) in cytology findings, cytology results of LSIL or higher, or HPV16 or HPV18 positivity.
Histopathological Examination
Cervical Biopsy
Cervical biopsy is a reliable method for diagnosing cervical intraepithelial lesions. Any suspicious lesion observed macroscopically or high-grade lesions identified under colposcopy should undergo single or multiple biopsies. Endocervical curettage (ECC) may be performed to assess lesions in the endocervical canal.
Diagnostic Cervical Conization
Diagnostic cervical conization is recommended in cases where multiple cytological assessments identify HSIL but no abnormalities are observed under colposcopy, or colposcopy findings are inadequate, or biopsy pathology results are negative. Conization is also advised for cases where cytology identifies atypical glandular cells favoring neoplasia (AGCFN) or AIS, but colposcopic biopsy results are ≤LSIL, or when cervical biopsy findings are insufficient to rule out invasive carcinoma.
Treatment
LSIL
LSIL generally does not require treatment and is managed through clinical observation with stratification based on cytological findings.
When the cytology result is ≤LSIL, follow-up observation is conducted at one-year intervals. If LSIL persists for two years or more, continued observation remains the first choice. Alternatively, diagnostic cervical conization or ablative treatment may be considered.
When the cytology result is HSIL, cytology, histopathology, and colposcopy findings are reviewed, with management based on the revised diagnosis. For cases in which the squamocolumnar junction (SCJ) and the upper border of the lesion are fully visible under colposcopy and endocervical curettage (ECC) reveals pathology less severe than CIN2, follow-up at intervals of 6–12 months may be undertaken. Diagnostic cervical conization may be performed to exclude precancerous lesions.
When the cytology result indicates atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), cytology, histopathology, and colposcopy findings are reviewed, with management based on the revised diagnosis. In cases where the SCJ and upper border of the lesion are fully visible under colposcopy and ECC reveals pathology less severe than CIN2, follow-up at intervals of 6–12 months may be conducted. Diagnostic conization is recommended if ASC-H persists for two years or if adequate follow-up monitoring is not feasible.
When the cytology result indicates atypical glandular cells, not otherwise specified (AGC-NOS), follow-up at intervals of 6–12 months is recommended after ruling out endometrial disease.
When the cytology result indicates atypical glandular cells, favor neoplastic (AGC-FN) or adenocarcinoma in situ (AIS), diagnostic cervical conization with intraoperative ECC should be performed.
HSIL
Treatment options are chosen based on pathological grading, individual preferences, and the resources of the medical institution. Cervical conization is recommended, including loop electrosurgical excision procedure (LEEP) or cold knife conization (CKC). In cases of CIN2 without cervical canal involvement and adequate colposcopic examination, ablation therapy may be considered but with caution.
For women with CIN2 who desire future fertility, follow-up observation at six-month intervals may be an option. If CIN3 is diagnosed during follow-up or CIN2 persists for two years, excisional surgery is required. For women with HSIL confirmed through cervical conization, who are older, have no fertility desire, or have indications for other gynecological benign disease surgeries, total hysterectomy may also be performed.
AIS
AIS lesions are often multifocal and discontinuous. For patients with AIS confirmed by biopsy, diagnostic cervical conization is used to exclude invasive adenocarcinoma. Total hysterectomy is the primary treatment choice. For patients desiring fertility and with negative surgical margins, follow-up observation may be considered.
HPV Vaccination
HPV vaccination is an effective and safe primary prevention measure to reduce HPV-related infections and diseases. Vaccination provides the greatest benefit to individuals aged 9–26 years. Despite vaccination, regular cervical cancer screening should still be performed.
Cervical Intraepithelial Lesion During Pregnancy
During pregnancy, elevated estrogen levels cause the columnar epithelium to extend outward to the vaginal portion of the cervix, and atypical hyperplasia can occur in the basal cells of the transformation zone. Immune function during pregnancy may also decrease, increasing susceptibility to HPV infection. Basal cell changes such as nuclear enlargement and hyperchromasia may occur in the transformation zone during pregnancy but generally resolve six weeks postpartum. Cytological examinations during pregnancy have a potential risk of misdiagnosis.
For HSIL and AIS diagnosed during pregnancy, invasive carcinoma should be ruled out. Only observation and follow-up are recommended during pregnancy, with reevaluation at 42 days postpartum to determine subsequent management based on the findings.