Vulvovaginal candidiasis (VVC), formerly known as candidal vulvovaginitis or candidal vaginitis, is a common form of vulvovaginitis caused by Candida species. Data indicate that approximately 75% of women experience at least one episode of VVC in their lifetime, and 45% may have two or more episodes.
Pathogens and Predisposing Factors
The causative organism is Candida albicans in 80–90% of cases, while non-albicans Candida species such as Candida glabrata, Candida parapsilosis, and Candida tropicalis account for 10–20% of infections. Candida proliferates in acidic environments, with a vaginal pH typically below 4.5. Although Candida has limited heat resistance and can be killed at 60°C within an hour, it exhibits greater resistance to factors such as desiccation, sunlight, ultraviolet radiation, and chemical agents.
Common precipitating factors include prolonged use of broad-spectrum antibiotics, pregnancy, diabetes mellitus, extensive use of immunosuppressants, and high-dose estrogen therapy. Contamination of the vagina by feces in cases of gastrointestinal Candida infections, wearing tight synthetic underwear, and obesity, which can elevate local vulvar temperature and moisture levels, are additional contributing factors.
Modes of Transmission
Infection primarily occurs through endogenous routes. As an opportunistic pathogen, Candida can colonize areas such as the vagina, oral cavity, and intestines, with transmission possible between these sites. Direct transmission may occur through sexual contact, while indirect transmission through contaminated clothing is less common.
Clinical Manifestations
Typical symptoms include vulvovaginal itching and an increase in vaginal discharge, which often appears as curd-like or cottage cheese-like. Pruritus can be intense, causing significant discomfort, particularly at night. Some patients may also experience burning pain in the vulva, dyspareunia, and dysuria, with the latter arising from urine irritating an edematous vulva. Vaginal discharge is characteristically thick, white, and curd-like.
On examination, the vulva may show redness and swelling, accompanied by scratch marks and, in severe cases, fissures, epithelial shedding, or even erosion. The vaginal mucosa and the inner surface of the labia minora may be covered with white, lumpy material. When this material is removed, the underlying mucosa appears erythematous. Acute cases may additionally present with erosion and superficial ulceration.
VVC can be classified as either uncomplicated or complicated, with the latter accounting for 10–20% of cases. Uncomplicated VVC includes sporadic episodes of mild or moderate VVC caused by Candida albicans in non-pregnant women. Complicated VVC includes episodes caused by non-albicans Candida species, severe VVC, recurrent VVC, pregnancy-associated VVC, or cases in special populations such as individuals with uncontrolled diabetes or immunosuppression. The severity of VVC can be assessed using a clinical scoring system, with a score of less than 7 indicating mild to moderate VVC and a score of 7 or higher indicating severe VVC.
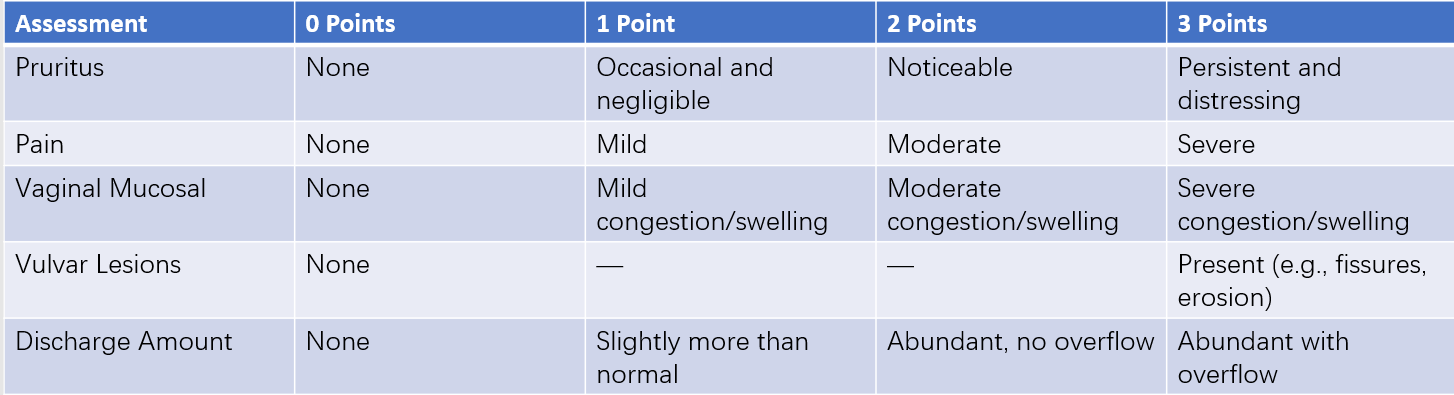
Table 1 Clinical scoring criteria for vulvovaginal candidiasis
Diagnosis
Diagnosis is confirmed by identifying budding Candida spores or pseudohyphae in vaginal discharge through microscopic examination. Vaginal discharge preparations can be examined using Gram-stained smears, 10% potassium hydroxide (KOH) wet mounts, or saline wet mounts, with sensitivities of 65–83%, 40–60%, and 30–50%, respectively. Gram-stained smears are considered the preferred diagnostic method. In symptomatic patients with negative microscopic findings, poor response to treatment, or recurrent cases, culture methods combined with antifungal susceptibility testing can be employed.
Attention should be given to possible co-infections with bacterial vaginosis and aerobic vaginitis. Measurement of pH can help distinguish infections; a pH below 4.5 suggests uncomplicated Candida infection, while a pH above 4.5 indicates the potential for mixed infections, particularly co-infections with bacterial vaginosis or aerobic vaginitis.
Treatment
The elimination of predisposing factors and the selection of either topical or systemic antifungal medications are determined based on the patient’s condition.
Elimination of Predisposing Factors
Discontinuation of medications such as broad-spectrum antibiotics and estrogen should occur promptly, and diabetes management should be optimized. Patients are advised to change underwear frequently, and previously used towels and other personal items should be sterilized by boiling.
Uncomplicated Vulvovaginal Candidiasis
Azole antifungal agents are commonly used in treatment.
Topical Therapy
Several options are available for intravaginal administration:
- Clotrimazole formulations: A single dose of 0.5g clotrimazole vaginal tablet; or clotrimazole suppository 0.15g nightly for 7 days; or 1% clotrimazole cream, 5g daily for 7–14 days.
- Miconazole formulations: A single dose of 1.2g miconazole; or 0.4g applied nightly for 3 days; or 0.2g applied nightly for 7 days.
- Nystatin: A dose of 100,000 units, applied nightly for 14 days.
Systemic Therapy
A single dose of 0.15g fluconazole is commonly utilized.
Complicated Vulvovaginal Candidiasis
Severe VVC
Treatment duration is doubled compared to therapy for uncomplicated VVC. For single-day oral or topical regimens, an additional dose is administered 72 hours after the initial treatment. If severe vulvar symptoms are present, a low-dose corticosteroid cream or azole cream may be applied to alleviate symptoms.
Recurrent VVC (RVVC)
This is defined as four or more episodes of VVC within a 12-month period, confirmed by mycological evidence. Treatment focuses on identifying and removing predisposing factors to prevent recurrence. Antifungal treatment involves two stages: induction therapy to achieve mycological cure, followed by maintenance therapy for six months.
Induction Therapy
Various intravaginal options are available:
- Clotrimazole vaginal tablets, 0.5g on days 1, 4, and 7.
- Miconazole, 1.2g on days 1, 4, and 7; or 0.4g nightly for 6 days; or nystatin, 100,000 units nightly for 14 days.
- Oral fluconazole, 0.15g on days 1, 4, and 7.
Maintenance Therapy
Although no universal protocol exists, oral fluconazole 0.15g weekly for six months is one option. Weekly use of miconazole (1.2g intravaginally), clotrimazole vaginal tablets (0.5g intravaginally), or nystatin (100,000 units nightly for 7 days before and after menstruation) for six months are alternative options.
Pregnancy-Associated VVC
Topical therapy with small doses over an extended period is preferred. Oral azole antifungal medications are contraindicated.
Considerations
Routine treatment of sexual partners is not required. For partners with balanitis or inflammation of the glans penis, Candida testing and treatment are necessary to prevent reinfection in women. For male partners with phimosis, daily cleaning is recommended, and elective surgical correction may be considered. For patients with recurring symptoms, the possibility of mixed vaginal infections or infections caused by non-albicans Candida should be evaluated.
Follow-Up
For patients with persistent symptoms or recurrence within two months post-treatment, follow-up is necessary. Fungal cultures with antifungal susceptibility testing may be performed. Patients with RVVC require follow-up evaluations at 7–14 days, one month, three months, and six months after completing treatment. Fungal cultures are recommended at the three- and six-month follow-up visits.