Hypertensive disorders of pregnancy (HDP) refer to a group of conditions characterized by elevated blood pressure during pregnancy, with an incidence rate of 5%–12%. This group includes gestational hypertension, preeclampsia, eclampsia, chronic hypertension with superimposed preeclampsia, and chronic hypertension in pregnancy. These conditions have a substantial impact on maternal and fetal health and represent a major cause of increased mortality and morbidity in pregnant women and perinatal infants.
Classification and Clinical Manifestations
The classification and clinical manifestations of hypertensive disorders of pregnancy are summarized in Table 1. Differences in pathophysiology and clinical management exist among preeclampsia–eclampsia, gestational hypertension, and chronic hypertension in pregnancy. This section primarily focuses on preeclampsia–eclampsia.
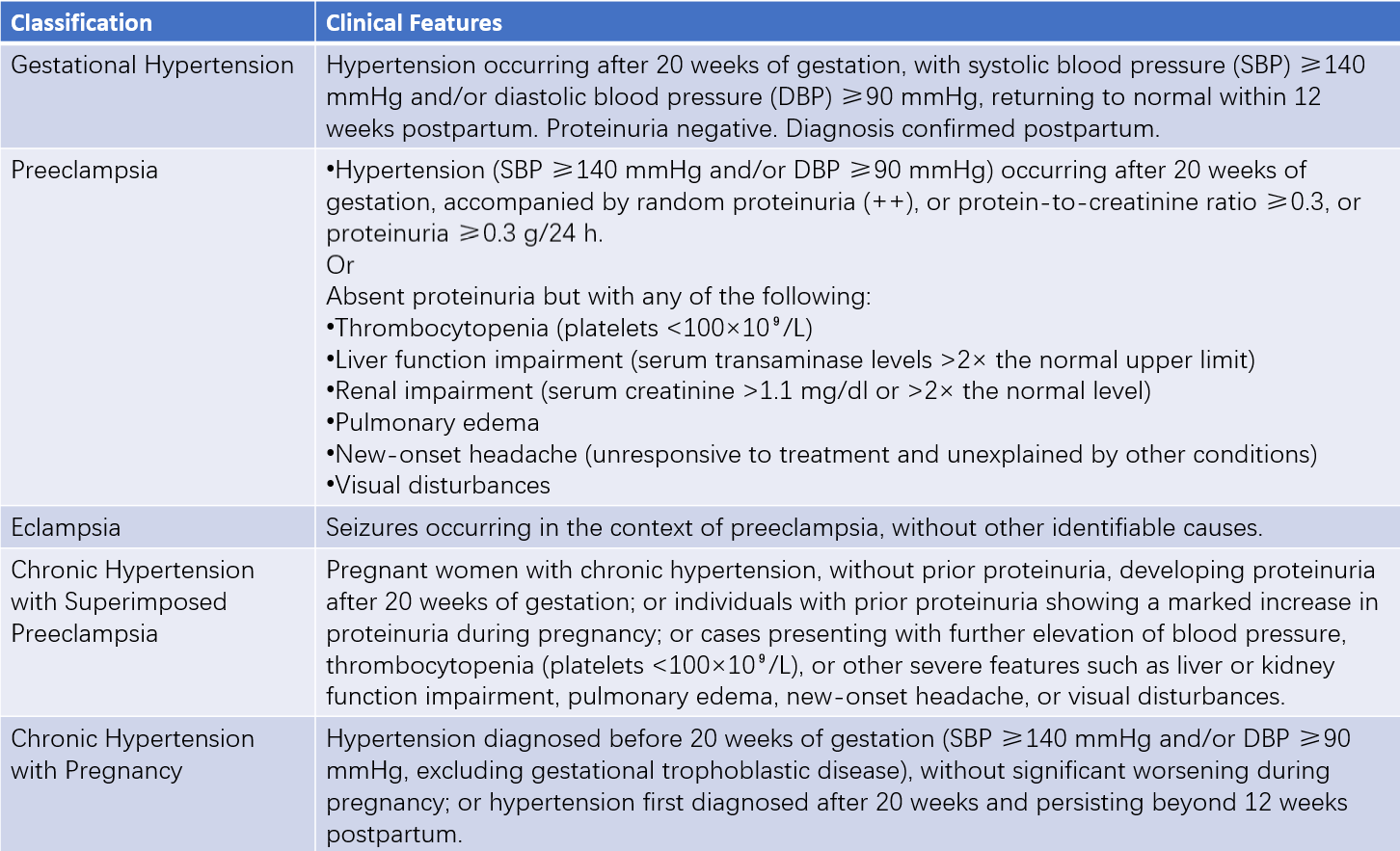
Table 1 Classification and clinical features of hypertensive disorders in pregnancy
Notes:
1, Early-onset preeclampsia is generally considered to occur before 34 weeks of gestation.
2, Severe proteinuria (≥5 g/24 h) is neither a criterion for assessing the severity of preeclampsia nor an indication for pregnancy termination, although close monitoring is required.
Preeclampsia–Eclampsia
Preeclampsia–eclampsia is a condition unique to pregnancy that occurs after 20 weeks of gestation. The disease is dynamic, with the potential for continuous progression of severity. The term "mild preeclampsia" only reflects the status at the time of diagnosis. Since preeclampsia of any severity can lead to serious adverse outcomes, the classification of "mild preeclampsia" is no longer used, and all cases are categorized as preeclampsia to avoid underestimating the severity of the condition. Preeclampsia with severe features (severe preeclampsia) is associated with significantly increased risks of adverse maternal and fetal outcomes, warranting heightened clinical attention. For diagnostic and management purposes, preeclampsia with severe features is referred to as "severe preeclampsia," and the diagnostic criteria include preeclampsia accompanied by any of the following:
- Systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg.
- Thrombocytopenia (platelet count <100 × 109/L).
- Liver dysfunction (serum transaminase levels more than twice the upper limit of normal), severe and persistent right upper quadrant or epigastric pain not attributable to other conditions, or both.
- Renal impairment (serum creatinine >1.1 mg/dL or doubling of creatinine concentration in the absence of other renal diseases).
- Pulmonary edema.
- New-onset headache unresponsive to medication and unexplained by other conditions.
- Visual disturbances.
Preeclampsia
Diagnosis
The diagnosis of preeclampsia is based on medical history, clinical manifestations, and auxiliary investigations. Due to the diverse clinical presentations of this condition, assessment of possible multi-organ involvement is essential.
Medical History
It is important to review any history of chronic hypertension, kidney disease, diabetes, systemic lupus erythematosus, thrombotic disorders, or family history of hypertensive disorders of pregnancy. Symptoms related to the current pregnancy—such as hypertension, proteinuria, headache, blurred vision, epigastric pain, oliguria, or seizures—should also be documented, along with the timing and severity of their onset.
Blood Pressure
Hypertension is defined as a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, based on measurements taken at least twice on the same arm. For cases where blood pressure has increased by 30/15 mmHg from baseline but remains below 140/90 mmHg, this does not meet the diagnostic criteria but calls for close monitoring. When elevated blood pressure is detected for the first time, it should be remeasured after an interval of at least 4 hours to confirm accuracy. Proper cuff size (length equal to 1.5 times the upper arm circumference) should be used for measurements. Severe hypertension (severe preeclampsia) is characterized by systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg, warranting clarification through prompt remeasurement.
Proteinuria
Proteinuria testing should be conducted at every antenatal check-up for high-risk pregnant women. Midstream urine samples are used for protein testing, and 24-hour urinary protein quantification is recommended for suspected preeclampsia. Diagnostic criteria for proteinuria include:
- Random urine protein ≥++
- Protein-to-creatinine ratio ≥0.3
- Protein ≥0.3 g/24 hours
Random urine protein tests are less accurate and should only be considered when quantitative testing is unavailable. Vaginal secretions or amniotic fluid contamination should be avoided, and differential diagnosis must consider urinary tract infections, severe anemia, heart failure, and dystocia-related proteinuria.
Auxiliary Examinations
Routine examinations should include the following:
- Complete blood count
- Urinalysis
- Coagulation profile
- Liver function tests
- Renal function tests
- Electrocardiogram
- Electronic fetal heart rate monitoring
- Ultrasonography to evaluate the fetus, placenta, and amniotic fluid
Additional tests may be conducted based on disease progression and clinical necessity, including:
- Fundoscopic examination
- Imaging studies (e.g., ultrasonography of the liver, gallbladder, pancreas, spleen, and kidneys)
- Electrolyte analysis
- Arterial blood gas analysis
- Cardiac Doppler ultrasonography and evaluation of cardiac function
- Doppler studies of the umbilical and uterine arteries
- Cranial CT or MRI
- Autoimmune disease-related markers, where available
Differential Diagnosis
The primary differential diagnosis for preeclampsia is chronic nephritis, as acute nephritis during pregnancy is less common. In cases where chronic nephritis preexists, proteinuria is often identified during pregnancy, and severe cases may exhibit casts and renal dysfunction, accompanied by sustained hypertension. Retinal changes associated with nephritis may also be present. Differentiating latent nephritis can be challenging and requires careful review of medical history and further assessment of glomerular and tubular renal function. Preeclampsia should also be distinguished from chronic hypertension that preexists pregnancy, as the latter manifests with hypertension prior to conception.
Etiology and Pathogenesis
The etiology and pathogenesis of preeclampsia remain incompletely understood. It is acknowledged as a multifactorial, multi-mechanism, and multi-pathway disease that defies explanation through a single unifying theory, contributing to its etiologic heterogeneity. A two-stage model has been proposed as a mechanism for the development of preeclampsia. The first stage, a preclinical phase, involves impaired remodeling of uterine spiral arteries, leading to placental ischemia and hypoxia and the release of multiple placental factors. The second stage involves the entry of placental factors into the maternal circulation, promoting systemic inflammatory activation and endothelial cell damage, which result in the diverse clinical manifestations associated with preeclampsia–eclampsia. Several key theories on the etiology and pathogenesis are outlined below.
Insufficient Remodeling of Uterine Spiral Arteries
Under normal pregnancy conditions, trophoblast cells differentiate into villous trophoblasts and extravillous trophoblasts (EVTs). EVTs include interstitial extravillous trophoblasts (iEVTs) and endovascular extravillous trophoblasts (enEVTs). The iEVTs invade the endometrial stroma up to the inner one-third of the myometrium, while the enEVTs migrate into the lumen of the uterine spiral arteries, gradually replacing vascular smooth muscle and endothelial cells. This process transforms the arteries from high-resistance, low-capacity vessels to low-resistance, high-capacity vessels, increasing placental blood flow to ensure efficient maternal-fetal material exchange and fetal development. In preeclampsia, the invasion capacity of extravillous trophoblasts is impaired, resulting in "shallow placentation" and severely insufficient remodeling of uterine spiral arteries. Only the decidual layer of the arteries undergoes remodeling, with the luminal diameter of the spiral arteries reduced to approximately half of normal pregnancies. This condition increases vascular resistance and decreases placental perfusion, triggering the clinical manifestations of preeclampsia. The mechanisms contributing to insufficient remodeling of uterine spiral arteries remain under investigation.
Excessive Activation of Inflammatory and Immune Responses
Excessive activation of inflammatory and immune responses is observed both locally at the maternal-fetal interface and systemically in preeclampsia patients. Current evidence suggests that the innate immune system, which dominates at the maternal-fetal interface, plays a significant role in preeclampsia pathogenesis. Abnormalities in the quantity, phenotype, and function of Toll-like receptor families, decidual natural killer (dNK) cells, and macrophages can disrupt the remodeling of uterine spiral arteries and contribute to shallow placentation. Specific immune mechanisms focus on T cells. During normal pregnancy, the maternal immune system shifts from a Th1 to a Th2 environment, whereas preeclampsia is associated with a local Th1 shift in the decidua. Regulatory T cells (Tregs), specifically CD4+ CD25+ T cells, play a role in regulating the Th1/Th2 balance. A marked reduction in Treg cells favors a Th1-dominant response, decreasing maternal immune tolerance of the fetus and contributing to preeclampsia.
Endothelial Cell Injury
Endothelial cell injury is a central pathological change in preeclampsia. This damage results in decreased synthesis of vasodilatory substances such as nitric oxide (NO) and prostacyclin (PGI2) and increased production of vasoconstrictive substances such as endothelin (ET) and thromboxane A2. These changes promote vasoconstriction and exacerbate the condition. Endothelial damage also activates platelets and coagulation factors, contributing to the hypercoagulable state characteristic of preeclampsia. Factors inducing endothelial cell injury include oxidative stress and inflammatory mediators, such as tumor necrosis factor, interleukin-6, and very low-density lipoproteins.
Genetic Factors
Preeclampsia exhibits familial tendencies, suggesting genetic factors are involved in its development, although the mode of inheritance remains unclear. The heterogeneity of preeclampsia, particularly the interaction of genetic and environmental factors, leads to complex phenotypes. While numerous susceptibility regions on chromosomes have been identified, significant challenges remain in pinpointing specific susceptibility genes within these regions.
Nutritional Deficiencies
Several nutritional deficiencies, such as hypoproteinemia and deficits in calcium, magnesium, zinc, and selenium, may be associated with the development and progression of preeclampsia. However, further clinical research is required to confirm these associations.
Pathophysiological Changes and Maternal-Fetal Impact
The primary pathophysiological changes in preeclampsia involve systemic small vessel vasospasm and endothelial cell injury. These changes reduce perfusion across multiple organs and systems, causing harm to both the mother and fetus, and in severe cases, may result in maternal and fetal death.
Brain
Cerebral vasospasm and increased vascular permeability lead to cerebral edema, congestion, localized ischemia, thrombosis, and hemorrhage. Computed tomography (CT) scans often reveal low-density areas in the cerebral cortex with corresponding ischemia and pinpoint hemorrhages, indicative of cerebral infarction. These findings are associated with symptoms such as coma, visual impairment, or blindness. Extensive cerebral edema can manifest as sensory dullness, confusion, or, in rare cases, coma and even brain herniation. Cerebral vascular resistance and cerebral perfusion pressure are elevated in preeclampsia, with high perfusion pressure contributing to severe headache. Eclampsia is related to the loss of cerebrovascular autoregulatory function.
Kidneys
Glomerular involvement includes glomerular dilation, endothelial cell swelling, and fibrin deposition in endothelial cells. Plasma proteins leak through the glomeruli, resulting in proteinuria. Renal blood flow and glomerular filtration rate decrease, causing elevated serum uric acid and creatinine levels. Severe renal impairment can result in oliguria or renal failure.
Liver
Liver damage commonly presents as elevated serum transaminase levels. The characteristic liver injury includes periportal hemorrhage, and in severe cases, periportal necrosis and subcapsular hematoma formation. Liver rupture may occur, posing a life-threatening risk to both the mother and fetus.
Cardiovascular System
Vascular spasm, elevated blood pressure, increased peripheral resistance, impaired myocardial contractility, and increased afterload (cardiac ejection resistance) significantly reduce cardiac output. The cardiovascular system presents a state of low output and high resistance. Additionally, endothelial cell activation increases vascular permeability, allowing intravascular fluid to leak into the myocardial interstitium. This leakage leads to myocardial ischemia, interstitial edema, myocardial microhemorrhage or necrosis, and pulmonary edema, and in severe cases, can result in heart failure.
Hematological Changes
Systemic arteriolar vasospasm and increased vascular permeability result in hemoconcentration and elevated hematocrit. A reduced hematocrit is often associated with anemia, red blood cell damage, or hemolysis.
Endocrine and Metabolic Changes
Increased angiotensin-converting enzyme levels and elevated aldosterone and deoxycorticosterone levels in late pregnancy lead to sodium and water retention. Plasma oncotic pressure decreases, and extracellular fluid volume exceeds that of normal pregnancy. However, the severity of edema has little correlation with the severity or prognosis of preeclampsia. Electrolyte levels are generally similar to those seen in normal pregnancy. Following eclamptic seizures, metabolic changes may include lactic acidosis and respiratory compensation through carbon dioxide loss, resulting in reduced serum bicarbonate concentrations.
Uteroplacental Blood Flow Perfusion
Insufficient remodeling of uterine spiral arteries reduces placental perfusion. The average diameter of uterine spiral arteries in preeclampsia is only half that observed in normal pregnancies. Furthermore, endothelial cell injury and acute atherosclerosis in placental vessels impair placental function, leading to intrauterine growth restriction and fetal distress. Placental abruption may occur if placental bed vessels rupture, with severe outcomes potentially causing maternal and fetal death.
Prediction and Prevention
Identifying preeclampsia risk factors is critical for early prevention and treatment to reduce maternal and perinatal mortality. However, no particularly effective, reliable, and cost-effective method of prediction currently exists. Risk assessment during the first prenatal visit is typically based on identified risk factors, and the use of multiple indicators may enhance prediction in facilities with appropriate resources.
Risk Prediction
Risk Factors
Epidemiological studies have identified significant risk factors associated with preeclampsia. Moderate-risk factors include primiparity, obesity (BMI ≥ 30 kg/m2), a family history of preeclampsia (in the mother or sisters), maternal age ≥ 35 years, and a personal history of adverse pregnancy outcomes such as low birth weight or intrauterine growth restriction. A previous adverse pregnancy outcome or an interpregnancy interval ≥ 10 years is also considered a moderate risk. High-risk factors include a history of preeclampsia (particularly with a prior adverse pregnancy outcome), multiple gestations, chronic hypertension, type 1 or type 2 diabetes, kidney disease, and autoimmune diseases (such as systemic lupus erythematosus or antiphospholipid syndrome).
Biochemical Markers
Biochemical markers with potential predictive value for preeclampsia include placental growth factor (PLGF), soluble Fms-like tyrosine kinase-1 (sFlt-1), placental protein 13 (PP13), and soluble endoglin (sEng). Combining these markers with risk factors may improve prediction accuracy.
Uterine Artery Doppler Flow Studies
Uterine artery Doppler flow studies performed between 11 and 13 weeks + 6 days of gestation provide predictive utility. Persistent elevations in the pulsatility index and resistance index, or abnormal diastolic notching in the uterine artery waveform, are pathologic findings associated with an increased risk of preeclampsia.
Preventive Measures
While no effective preventive strategies exist for low-risk populations, certain measures may benefit high-risk individuals identified through prediction.
Moderate Exercise
Maintaining appropriate physical activity levels during pregnancy and arranging sufficient rest promotes overall maternal health.
Nutritional Considerations
Severe salt restriction is not recommended during pregnancy, nor is caloric restriction in obese pregnant individuals.
Calcium Supplementation
Pregnant women with low calcium intake (<600 mg/day) may benefit from calcium supplementation, typically 1.5–2.0 g per day.
Low-Dose Aspirin
For individuals at high risk for preeclampsia, low-dose aspirin may be an effective prophylactic measure. Administration begins between 11 and 13 weeks + 6 days of gestation, no later than 20 weeks, with a daily dosage of 100–150 mg at bedtime. Aspirin use continues until 36 weeks or 5–10 days prior to the anticipated delivery date.
Treatment
The goal of treatment is to control the disease, prolong gestation, and ensure the safety of both the mother and fetus as much as possible. The primary principles of treatment include blood pressure control, antispasmodic therapy, and sedation, as well as close monitoring of maternal and fetal conditions. Timely termination of pregnancy remains the most effective intervention.
Assessment and Monitoring
The course of preeclampsia is complex and progresses rapidly. Physiological changes associated with labor and the postpartum period, alongside various adverse stimuli, may exacerbate the condition. Close and dynamic monitoring of the mother's condition before, during, and after delivery is necessary to understand disease progression, implement timely and appropriate interventions, and reduce the likelihood of adverse clinical outcomes. The scope and frequency of evaluation depend on the severity of the condition.
The content of assessment and monitoring includes:
- Attention to symptoms such as headache, blurred vision, chest tightness, abdominal pain, vaginal bleeding, edema, and oliguria.
- Comprehensive laboratory and auxiliary investigations, including routine blood tests, urinary protein assessment, coagulation function, liver and kidney function tests, echocardiography, electronic fetal heart rate monitoring, obstetric ultrasound, and umbilical artery blood flow studies.
- Dynamic monitoring of changes in maternal blood pressure, weight, urine output, and fetal growth and development.
General Management
Outpatient treatment may be sufficient for non-severe preeclampsia patients, while hospitalization is required for those with severe preeclampsia.
Adequate rest should be maintained, with sufficient intake of protein and calories. Salt restriction is not recommended.
Adequate sleep should be ensured. If necessary, 2.5–5 mg of diazepam may be taken orally before bedtime.
Antihypertensive Therapy
The purpose of antihypertensive treatment is to prevent complications such as eclampsia, cardiovascular or cerebrovascular events, and placental abruption. Severe hypertension, defined as systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 110 mmHg, necessitates aggressive antihypertensive treatment. Non-severe hypertension, defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, may warrant treatment, particularly when accompanied by organ dysfunction.
Target blood pressure levels are recommended within the range of 130–139 mmHg for systolic pressure and 80–89 mmHg for diastolic pressure. The reduction in blood pressure should be gradual and steady, avoiding excessive fluctuations, with an ideal target of approximately 130/80 mmHg.
Oral antihypertensive medications are commonly used. If oral therapy is insufficient, intravenous medications can be administered. Diuretics are generally avoided during pregnancy to prevent hemoconcentration, reductions in effective circulating blood volume, and hypercoagulability. Beta blockers such as atenolol and alpha-blockers like prazosin are not recommended, and both angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-II receptor blockers (ARBs) are contraindicated during pregnancy. Commonly used antihypertensive medications include the following:
Labetalol
Labetalol is an α and β receptor antagonist that lowers blood pressure without affecting renal or placental blood flow. It also counteracts platelet aggregation and promotes fetal lung maturation. The drug has a rapid onset of action and does not cause excessive hypotension or reflex tachycardia.
- Oral: 50–150 mg, 3–4 times daily.
- Intravenous injection: Initial dose of 20 mg; if blood pressure remains uncontrolled after 10 minutes, the dose may be doubled, with a maximum single dose of 80 mg, until blood pressure is controlled.
- Intravenous infusion: Add 50–100 mg to 250–500 ml of 5% glucose, adjusting the infusion rate according to blood pressure. Once stabilized, switch to oral medication.
Nifedipine
Nifedipine is a calcium channel blocker that relieves peripheral vasospasm, leading to systemic vasodilation and blood pressure reduction. Due to its rapid hypotensive effect, sublingual administration is generally not recommended.
- Oral: 10 mg every 6–8 hours. Dosage may be increased if necessary, ranging from 30–90 mg per day, with a maximum total daily dose of 120 mg.
- Side effects include palpitations and headache. Blood pressure changes should be closely monitored to avoid hypotension-related complications.
Methyldopa
Methyldopa acts by stimulating α-receptors in the vasomotor center, suppressing sympathetic outflow to reduce blood pressure. It is effective during pregnancy.
- Oral: 250 mg, 3–4 times daily. The dose may be adjusted based on clinical conditions, with a maximum daily dose of 2 g.
- Side effects include drowsiness, constipation, dry mouth, and bradycardia.
Nicardipine
Nicardipine is a dihydropyridine calcium channel blocker.
- Oral: Initial dose of 20–40 mg, three times daily.
- Intravenous infusion: Start with 1 mg/h and adjust the dose every 10 minutes based on blood pressure.
Urapidil
Urapidil is an α-receptor antagonist with effects on both central and peripheral mechanisms, providing rapid blood pressure reduction.
- Intravenous injection: 10–50 mg via slow intravenous injection over 5 minutes. If the response is insufficient, repeat after 5 minutes.
- Intravenous infusion: Administer at 2 mg/min, adjusting the infusion rate as needed, with a maintenance rate of 9 mg/h. Orthostatic hypotension is a potential side effect. Adequate time intervals should be observed if prior antihypertensives were used, and dosage adjustments may be required.
Nitroglycerin
Nitroglycerin acts on nitric oxide synthase to dilate both arteries and veins, reducing both preload and afterload. It is primarily used in hypertensive emergencies associated with heart failure or acute coronary syndromes.
- Intravenous infusion: Start at 5–10 μg/min and increase the rate every 5–10 minutes to a maintenance dose of 20–50 μg/min.
Phentolamine
Phentolamine is an α-receptor antagonist.
- Dosage: 10–20 mg diluted in 100–200 ml of 5% glucose, administered by intravenous infusion at a rate of 10 μg/min.
Sodium Nitroprusside
Due to potential toxic effects of its metabolites on both the mother and fetus, sodium nitroprusside is reserved for severe hypertension unresponsive to other medications.
- Dosage: 50 mg dissolved in 500 ml of 5% glucose, administered via intravenous infusion at a rate of 0.5–0.8 μg/(kg·min). Blood pressure and heart rate require close monitoring during administration.
Antispasmodic Therapy
Magnesium sulfate is considered the first-line medication for treating eclampsia and is a key medication for preventing eclamptic seizures in severe preeclampsia. It has been shown to be more effective in controlling recurrent seizures compared to sedative medications such as diazepam, sodium phenobarbital, or hibernation mixtures. Unless contraindications exist or the treatment proves ineffective, magnesium sulfate is preferred over alternatives like diazepam or phenytoin for the prevention or treatment of eclampsia.
Mechanism of Action
Magnesium ions alleviate spasms through several mechanisms:
- Inhibition of acetylcholine release: This action blocks signal transduction at the neuromuscular junction, leading to skeletal muscle relaxation.
- Stimulation of vascular endothelial production of prostacyclin: This reduces the synthesis of endothelin and reactivity to angiotensin II, thereby alleviating vascular spasm.
- Blocking glutamate channels: This prevents calcium influx, relieving vascular spasm and reducing endothelial cell damage.
- Enhanced oxygen metabolism: It increases the affinity of hemoglobin for oxygen in both the mother and fetus.
Indications for Use
- Controlling eclamptic seizures and preventing recurrence.
- Preventing the progression of severe preeclampsia into eclampsia.
- Administering prior to delivery in severe preeclampsia to prevent intrapartum or postpartum eclampsia.
- Magnesium sulfate is not to be used as an antihypertensive agent.
Principles of Use
- The same regimen is used for both prevention and treatment of eclampsia.
- It can be initiated during labor in patients who have not received magnesium sulfate prior to delivery, and continued for at least 24–48 hours postpartum.
- Maintaining a stable serum magnesium concentration is essential.
Dosing Regimen
- Intravenous administration: A loading dose of 4–6 g of magnesium sulfate is diluted in 20 ml of 25% glucose and given via slow intravenous injection over 15–20 minutes. Alternatively, it can be diluted in 100 ml of 5% glucose for rapid intravenous infusion over 15–20 minutes. Maintenance doses of 1–2 g per hour are administered via intravenous infusion.
- Intramuscular administration: If better nighttime sleep is required, intravenous medication may be discontinued before bedtime and replaced with a single intramuscular injection of 20 ml of 25% magnesium sulfate combined with 2 ml of 2% lidocaine, injected deeply into the gluteal muscle.
- The total dose of magnesium sulfate within 24 hours should not exceed 30 g, and the duration of treatment should generally not exceed five days.
Precautions
- The effective therapeutic serum magnesium concentration ranges from 1.8 to 3.0 mmol/L. Toxicity symptoms may occur at levels exceeding 3.5 mmol/L.
- The use of magnesium sulfate requires the following conditions to be met:
- Presence of patellar reflexes.
- Respiratory rate of ≥16 breaths per minute.
- Urine output of ≥17 ml/h or ≥400 ml/24 h.
- Availability of 10% calcium gluconate for use as an antidote.
- In the event of magnesium toxicity, magnesium sulfate should be discontinued, and 10 ml of 10% calcium gluconate should be administered slowly via intravenous injection over 5–10 minutes.
- Magnesium sulfate should be used with caution or at reduced doses in patients with concurrent renal dysfunction, cardiomyopathy, or myasthenia gravis. Serum magnesium concentrations may be monitored if the necessary resources are available.
Sedation
Sedative medications can relieve maternal psychological stress, anxiety, and improve sleep. When magnesium sulfate is ineffective or contraindicated, sedatives can be used to prevent and control eclampsia.
Diazepam
Diazepam provides strong sedative, anticonvulsant, and muscle relaxant effects with minimal impact on the fetus and neonate.
- Dosing: 2.5–5 mg orally, three times daily or once before bedtime; 10 mg via intramuscular injection or slow intravenous injection (>2 minutes) can be used to prevent eclampsia. Respiratory depression may occur if doses exceed 30 mg within one hour.
Hibernation Mixture
This combination of medications acts as a central nervous system depressant, helping to alleviate spasms, reduce blood pressure, and control eclamptic seizures. The mixture typically consists of 100 mg of pethidine, 50 mg of chlorpromazine, and 50 mg of promethazine.
- Administration: One-third or one-half of the dose may be injected intramuscularly, or the entire dose may be diluted in 250 ml of 5% glucose and infused slowly intravenously.
Due to the risk of chlorpromazine-induced rapid blood pressure drops, which can reduce renal and uteroplacental blood flow and lead to fetal hypoxia, as well as hepatic toxicity to both mother and fetus, its use is generally limited to cases where magnesium sulfate is ineffective.
Sodium Phenobarbital
Sodium phenobarbital is effective for sedation, anticonvulsant activity, and control of seizures.
- Dosing: During eclamptic seizures, 0.1 g may be given via intramuscular injection. For prevention, 30 mg per dose may be taken orally three times daily.
Due to the potential for respiratory depression in the fetus, its use is avoided within six hours before delivery.
Diuretics
Routine use of diuretics is not recommended. In cases of generalized edema, pulmonary edema, cerebral edema, renal dysfunction, or acute heart failure, the use of fast-acting diuretics such as furosemide may be considered on a case-by-case basis. Mannitol is primarily used for cerebral edema; however, it is contraindicated in patients with heart failure or impending heart failure due to its hyperosmotic diuretic properties. Fructose-containing agents may be suitable for patients with renal impairment. For patients with severe hypoalbuminemia and ascites, albumin supplementation can be administered prior to the use of diuretics.
Promotion of Fetal Lung Maturity
For preeclampsia patients with gestational age < 34 weeks, corticosteroid therapy for promoting fetal lung maturity should be administered if delivery is anticipated within one week. For pregnancies between 34 and 36 weeks, there is some controversy regarding the use of corticosteroids, and decisions should be made by weighing the risks and benefits with informed consent.
Timing and Mode of Delivery
When maternal or fetal conditions do not improve or the disease continues to progress despite active treatment, terminating the pregnancy becomes the only effective intervention.
Timing of Termination
For patients with non-severe preeclampsia, expectant management until 37 weeks is generally recommended, after which delivery may be induced.
For patients with severe preeclampsia:
- If gestational age is < 24 weeks and the condition remains unstable after treatment, termination of pregnancy is advised.
- For gestational ages between 24–27 weeks, decisions on expectant management depend on maternal and fetal conditions, as well as local medical resources and expertise.
- For gestational ages of 28–33 weeks, termination of pregnancy is indicated when the condition remains unstable or worsens after 24–48 hours of aggressive treatment, after promoting fetal lung maturity. If the condition stabilizes, further expectant management may be possible, and transfer to a facility with advanced neonatal care capabilities is recommended.
- For gestational ages ≥ 34 weeks, delivery is typically considered.
Mode of Delivery
In the absence of indications for cesarean delivery, vaginal delivery is generally preferred.
If vaginal delivery cannot be achieved within a short period and the maternal condition risks worsening, the indications for cesarean section may be broadened.
Intrapartum Considerations
Close monitoring of maternal symptoms, including subjective symptoms and blood pressure changes, is necessary. Blood pressure control should aim for levels ≤ 160/110 mmHg, and antihypertensive therapy should be continued.
Fetal heart rate monitoring is required.
Active measures to prevent postpartum hemorrhage may be implemented.
Postpartum Management
In preeclampsia patients, particularly those with severe preeclampsia, magnesium sulfate therapy is recommended to continue for 24–48 hours postpartum to prevent the occurrence of postpartum eclampsia. During the postpartum period (7–10 days), there is a peak in blood pressure fluctuations, and symptoms such as hypertension and proteinuria may persist or even worsen. Daily monitoring of blood pressure is necessary, and antihypertensive therapy should be considered when blood pressure levels reach ≥ 140/90 mmHg. Persistent postpartum hypertension requires evaluation and investigation to rule out other systemic diseases.
Management of Early-Onset Severe Preeclampsia
Severe preeclampsia that occurs prior to 34 weeks of gestation is referred to as early-onset preeclampsia, while cases occurring at or after 34 weeks are classified as late-onset preeclampsia. Although both share some clinical characteristics, their pathophysiological mechanisms and prognoses differ. Early-onset severe preeclampsia presents more severe clinical manifestations and is associated with poorer pregnancy outcomes, necessitating more rigorous monitoring and assessment.
Hospitalization is advised for patients with early-onset severe preeclampsia. Treatment typically includes magnesium sulfate for seizure prophylaxis, antihypertensive therapy, and corticosteroids to promote fetal lung maturity. Close monitoring of maternal and fetal conditions is essential, with comprehensive assessment to identify potential severe organ dysfunction that may warrant termination of pregnancy. Termination of pregnancy may be considered under the following circumstances:
- Persistent maternal symptoms or severe hypertension.
- Eclampsia, pulmonary edema, or HELLP syndrome.
- Severe renal dysfunction or coagulation disorders.
- Placental abruption.
- Non-viable fetus due to extremely early gestational age.
- Signs of fetal distress.
Eclampsia
Eclampsia represents the most severe stage of the preeclampsia-eclampsia spectrum. Severe symptoms that progressively worsen may precede its onset, though it may also occur in patients without significant blood pressure elevation or proteinuria. Eclampsia typically occurs during the antenatal period, with approximately 25% of cases reported within 48 hours postpartum. The rapid progression of eclamptic seizures is a primary cause of maternal and fetal mortality, necessitating prompt intervention.
Clinical Presentation
The prodromal phase is brief and characterized by convulsions, facial flushing, frothing at the mouth, and deep coma. This is followed by rigidity of deep muscles, rapidly developing into typical generalized tonic-clonic convulsions with rhythmic muscle contractions and tension. The seizures last approximately 1 to 1.5 minutes, during which respiratory movements cease. After the seizure subsides, breathing resumes, but the patient remains comatose. Consciousness is eventually restored, but irritability and agitation may persist.
Diagnosis and Differential Diagnosis
Eclampsia is often diagnosed based on convulsions occurring in the context of preeclampsia. However, it must be differentiated from other conditions such as epilepsy, encephalitis, brain tumors, ruptured cerebral vascular malformations with intracranial hemorrhage, hyperosmolar diabetic coma, and hypoglycemic coma. A thorough medical history and diagnostic evaluation typically enable differentiation.
Treatment
General Emergency Management
During eclamptic seizures, maintaining airway patency and stabilizing respiratory and circulatory functions are critical. Close monitoring of vital signs, placement of a urinary catheter to monitor urine output, and control of environmental stimuli (e.g., noise and light) are advised. Measures to prevent injuries from falls or trauma, as well as lip and tongue biting, are also necessary.
Seizure Control
Magnesium sulfate remains the first-line treatment for eclampsia and prevention of seizure recurrence. For patients with contraindications to magnesium sulfate or those who do not respond to it, alternatives such as diazepam, phenytoin, or hibernation mixtures may be used to control seizures. Postpartum use of magnesium sulfate is recommended for 24–48 hours.
Reduction of Intracranial Pressure
Rapid intravenous infusion of 250 ml of 20% mannitol may be utilized to reduce intracranial pressure.
Blood Pressure Control
Cerebrovascular events are the most common cause of death in eclamptic patients. Persistent systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 110 mmHg requires active antihypertensive treatment to prevent cerebrovascular complications.
Correction of Hypoxia and Acidosis
Oxygen delivery via mask and bag is recommended. Acid-base imbalances, including acidosis, may be corrected based on arterial blood gas parameters such as pH, partial pressure of carbon dioxide (pCO2), and bicarbonate concentration using appropriate doses of 4% sodium bicarbonate.
Termination of Pregnancy
Once convulsions are controlled, termination of pregnancy should be considered.
Other Types of Hypertensive Disorders in Pregnancy
In addition to preeclampsia-eclampsia, hypertensive disorders in pregnancy also include gestational hypertension, chronic hypertension with pregnancy, and chronic hypertension complicated by superimposed preeclampsia. The principles of assessment and management for the latter three conditions are outlined below.
Gestational Hypertension
Assessment and Monitoring
Gestational hypertension is diagnosed when hypertension first appears after 20 weeks of gestation, is not accompanied by proteinuria, and resolves with normal blood pressure levels by 12 weeks postpartum. Over 50% of patients may progress to preeclampsia, necessitating close monitoring and dynamic evaluation throughout pregnancy.
Treatment
The primary approach involves antihypertensive therapy, with treatment goals and choice of antihypertensive medications aligned with preeclampsia management. For patients with stable maternal and fetal conditions, expectant management up to 37 weeks of gestation or later is permissible, followed by delivery.
Chronic Hypertension with Pregnancy
Assessment and Monitoring
Chronic hypertension in pregnancy increases maternal and fetal risks, including placental abruption and fetal growth restriction. Additionally, 13% to 40% of patients may develop superimposed preeclampsia. Strengthened monitoring and evaluation during pregnancy is necessary, including:
- Initial assessment of known or suspected cases of chronic hypertension in pregnant women.
- Referral to a specialized hypertension clinic is recommended if patients present with refractory hypertension, serum potassium levels < 3.0 mmol/L, serum creatinine levels > 97.2 μmol/L, or a family history of kidney disease.
- For patients with poorly controlled blood pressure, intensified blood pressure monitoring is necessary. If "white-coat hypertension" is suspected, dynamic blood pressure monitoring may be used to confirm the diagnosis prior to initiating antihypertensive therapy.
- Fetal growth and intrauterine well-being should be monitored to promptly identify fetal growth restriction and implement appropriate interventions.
Treatment
The primary treatment goal is to minimize the maternal and fetal risks of hypertension while prolonging pregnancy as safely as possible. The treatment principles are as follows:
- Antihypertensive goals and medication selection are consistent with those for preeclampsia.
- Time to delivery depends on the presence of complications. In the absence of complications, pregnancy is typically terminated between 38 to 39 weeks of gestation.
Chronic Hypertension with Superimposed Preeclampsia
Assessment and Monitoring
Chronic hypertension is frequently complicated by superimposed preeclampsia, which poses significantly higher risks to both the mother and fetus. Patients with chronic hypertension require vigilant monitoring for the development of severe preeclampsia, which, if present, is managed according to the protocol for preeclampsia.
Treatment
For patients with chronic hypertension complicated by superimposed preeclampsia, expectant management up to 37 weeks is acceptable if maternal and fetal conditions remain stable, with delivery planned thereafter. If severe preeclampsia develops, management follows the guidelines for severe preeclampsia as previously described.