A series of physiological changes occur in various maternal systems during pregnancy under the influence of hormones produced by the placenta and neuroendocrine regulation. These changes aim to support fetal growth and development and prepare the body for labor.
Changes in the Reproductive System
Uterus
The uterus plays a critical role during pregnancy in accommodating the embryo and fetus, and it is a key organ during labor. It is the organ that undergoes the most significant changes during and after pregnancy.
Uterine Size
As pregnancy progresses, with the formation and development of the fetus, placenta, and amniotic fluid, the uterine body gradually enlarges and softens. By full term, the uterus measures approximately 35 cm × 25 cm × 22 cm in size, with a capacity of about 5,000 ml, which is 500–1,000 times greater than its non-pregnant state. Its weight increases to approximately 1,100 g, nearly 20 times its original mass. In early pregnancy, the uterus appears slightly spherical and asymmetrical, with the uterine wall at the implantation site of the fertilized egg prominently thickened. After 12 weeks of pregnancy, the enlarged uterus gradually extends beyond the pelvic cavity and can be palpated above the pubic symphysis. In late pregnancy, the uterus rotates slightly to the right, associated with the positioning of the sigmoid colon on the left side of the pelvis. Uterine enlargement primarily results from hypertrophy and elongation of muscle cells, along with a slight increase in muscle cell numbers and connective tissue proliferation. Uterine myocytes grow from approximately 20 μm in length and 2 μm in width in the non-pregnant state to 500 μm in length and 10 μm in width at full term. These cells become rich in contractile proteins, such as actin and myosin, which provide the foundation for uterine contractions during labor. The thickness of the uterine myometrium is about 1 cm in the non-pregnant state, increases to 2.0–2.5 cm in mid-pregnancy, and then thins to 1.0–1.5 cm by the end of pregnancy. Uterine enlargement in early pregnancy is predominantly influenced by estrogen, while the role of progesterone remains less clear. After 12 weeks of pregnancy, uterine enlargement is attributed to increased intrauterine pressure. The growth rate of different uterine regions varies: the fundus grows the fastest in late pregnancy, the body has the highest muscle fiber content, the lower uterine segment grows less, and the cervix grows the least. These changes accommodate the pattern of uterine contractions during labor, where the contractile force gradually decreases from the fundus to the lower segments, facilitating fetal expulsion.
Irregular, painless contractions can occur in the uterus from early pregnancy. These contractions are infrequent, irregular, and asymmetrical, and they gradually increase as pregnancy progresses. The intrauterine pressure during such contractions typically ranges from 5 to 25 mmHg, lasting less than 30 seconds without cervical dilatation. This type of physiological, painless uterine contraction is referred to as Braxton Hicks contractions, also known as "false" contractions.
Uterine Blood Flow
During pregnancy, uterine blood vessels dilate and thicken, resulting in an increased uterine blood flow to meet the needs of the fetal-placental circulation. Uterine blood flow is approximately 50 ml/min in early pregnancy, primarily supplying the uterine myometrium and decidua. By full term, uterine blood flow increases to 450–650 ml/min, with 80–85% directed to the placenta. Uterine blood vessels run between myometrial muscle fibers, and blood flow significantly decreases during uterine contractions due to vessel compression. Excessively strong contractions can result in fetal hypoxia in utero. However, effective uterine contractions are also essential postpartum to help control hemorrhage by promoting rapid hemostasis at the placental detachment site.
Endometrium
After implantation of the fertilized egg, progesterone and estrogen stimulate significant changes in the endometrium, including enlargement of the glands, an increase in glycogen within glandular epithelial cells, hypertrophy of connective tissue cells, and engorgement of blood vessels. During this period, the endometrium is referred to as the decidua. Based on its relationship with the blastocyst, the decidua is divided into three parts:
- Basal decidua: The portion of the endometrium at the implantation site, associated with the chorion frondosum, which later develops into the maternal part of the placenta.
- Capsular decidua: The portion of the decidua covering the surface of the blastocyst, which protrudes into the uterine cavity as the blastocyst develops.
- True decidua: The remainder of the decidua that lines the uterine cavity, excluding the basal and capsular decidua. By 14–16 weeks of pregnancy, the amniotic cavity enlarges significantly, bringing the capsular and true decidua into close contact, thus obliterating the uterine cavity.
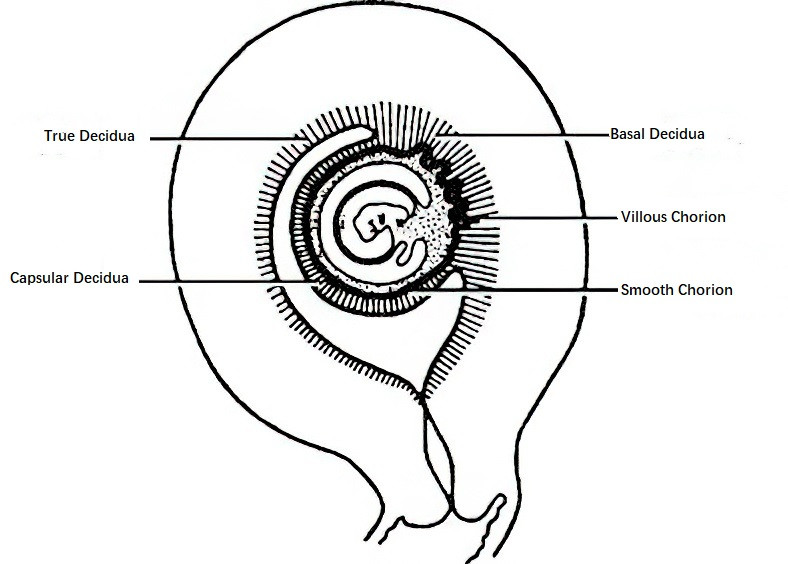
Figure 1 Relationship between decidua and villi in early pregnancy
Isthmus of the Uterus
The uterine isthmus, located between the uterine body and cervix, is the narrowest part of the uterus. Its length is about 1 cm in the non-pregnant state. During pregnancy, the isthmus softens, stretches, and thins, eventually becoming part of the uterine cavity. By labor, it extends to a length of 7–10 cm and forms part of the birth canal, referred to as the lower uterine segment.
Cervix
Under the influence of hormones, the cervix becomes engorged, edematous, and its endocervical glands proliferate and enlarge. As early as the first trimester, the cervix gradually softens and appears bluish-purple in color. The cervix primarily consists of connective tissue rich in collagen, and its remodeling during pregnancy allows it to remain closed until term, to dilate during labor, and to recover rapidly postpartum. Mucus production increases during pregnancy, forming a viscous mucus plug that is rich in immunoglobulins and cytokines, providing protection against ascending infections into the uterine cavity.
Ovaries
During pregnancy, ovulation and the development of new follicles cease. Up until 6–7 weeks of gestation, the ovaries produce large amounts of estrogen and progesterone to sustain the pregnancy. After 10 weeks of gestation, the placenta assumes the function of hormone production, leading to the regression of the corpus luteum.
Fallopian Tubes
During pregnancy, the fallopian tubes elongate, but their muscular layer does not thicken. The epithelial cells of the mucosal layer become somewhat flattened, and decidual cells can be observed in the stroma. Occasionally, the mucosa exhibits decidual-like changes.
Vagina
During pregnancy, the vaginal mucosa becomes softer and exhibits edema and congestion, appearing purplish-blue (Chadwick's sign). The rugae of the vaginal wall increase, the surrounding connective tissue becomes more loosened, the muscle cells hypertrophy, and extensibility improves, facilitating the passage of the fetus during delivery. Vaginal desquamated cells and secretions increase, appearing as a white, mucus-like substance. Glycogen levels in the vaginal epithelial cells rise, leading to increased lactic acid production and a lowered vaginal pH, which inhibits the growth of pathogenic bacteria and helps prevent infection.
External Genitalia
During pregnancy, the external genitalia experience congestion and thickening of the skin. Pigmentation of the labia majora and labia minora increases, while blood vessels in the labia majora become more numerous, and the connective tissue softens, enhancing extensibility for the passage of the fetus during delivery. As a result of compression by the enlarged uterus, venous return from the pelvis and lower limbs may be impaired, leading to varicose veins in the external genitalia or lower limbs in some pregnant individuals. These changes often resolve spontaneously postpartum.
Changes in the Breasts
During pregnancy, large amounts of estrogen secreted by the placenta stimulate the development of mammary ducts, while progesterone stimulates the development of mammary alveoli. Complete mammary development also requires the involvement of prolactin, placental lactogen, insulin, and cortisol. From early pregnancy, the breasts enlarge, and significant engorgement occurs. Breast fullness or tenderness is a common early symptom of pregnancy. As the mammary alveoli proliferate, the breasts enlarge further, and nodules may become noticeable. The nipples enlarge, darken, and exhibit increased erectility. The areola also darkens, and the sebaceous glands around its periphery enlarge, forming scattered nodular elevations known as Montgomery's tubercles.
Near the end of pregnancy, especially close to delivery, gentle expression of the breast may produce a small amount of thin, pale-yellow fluid known as colostrum. While the mammary glands undergo full development for lactation during pregnancy, milk secretion does not occur, likely due to the inhibitory effects of high levels of estrogen and progesterone. Following delivery of the placenta, levels of these hormones drop sharply, and nipple stimulation by the newborn triggers milk production.
Changes in the Circulatory System
Heart
The enlarged uterus during pregnancy raises the diaphragm, causing the heart to shift upward, leftward, and forward. The heart also rotates clockwise along its longitudinal axis. With the increase in blood volume and blood flow velocity, the cardiac dullness area slightly expands, and the point of maximal impulse shifts 1–2 cm to the left. Some pregnant individuals may exhibit Grade I–II soft, blowing systolic murmurs, splitting of the first heart sound, and a third heart sound, which typically resolve post-delivery. On an electrocardiogram, a leftward deviation of the electrical axis (approximately 15°) may be observed due to the leftward displacement of the heart. By late pregnancy, cardiac size increases by approximately 10%, and the resting heart rate rises by 10–15 beats per minute.
Cardiac Output
Accompanying the decrease in peripheral vascular resistance, increased heart rate, and expanded blood volume, cardiac output begins rising from 8–10 weeks of gestation, peaking at 32–34 weeks, and remaining elevated until delivery. In the left lateral decubitus position, cardiac output increases by approximately 30% compared to the non-pregnant state. The rise in cardiac output is the most significant adaptation of the circulatory system during pregnancy, ensuring adequate blood supply to the uterus, placenta, and breasts. Cardiac output also increases significantly during the second stage of labor. Pregnant individuals with pre-existing heart disease are at higher risk for heart failure during both pregnancy and delivery.
Blood Pressure
Blood pressure tends to be lower in early and mid-pregnancy, rising slightly after 24–26 weeks of gestation. Generally, systolic blood pressure remains unchanged, while diastolic blood pressure decreases slightly due to peripheral vasodilation, hemodilution, and the creation of arteriovenous shunts in the placenta, leading to a slightly widened pulse pressure. Maternal posture affects blood pressure; in late pregnancy, the supine position may compress the inferior vena cava, reducing venous return and cardiac output, leading to a drop in blood pressure—a condition known as supine hypotensive syndrome. The lateral decubitus position relieves uterine compression and improves venous return. Thus, lateral positioning is beneficial for rest during mid- to late pregnancy.
A significant increase in venous pressure in the lower limbs occurs during pregnancy. Together with compression of the inferior vena cava by the enlarged uterus, this can lead to lower limb edema, varicose veins, and an increased incidence of hemorrhoids. These conditions also raise the risk of deep vein thrombosis (DVT).
Changes in Blood
Blood Volume
Blood volume increases during pregnancy to meet the heightened blood supply required by the uterus, placenta, and other tissues and organs. This adaptation is critical for supporting fetal growth and development and serves as a protective mechanism against blood loss during pregnancy and delivery. Blood volume begins to rise at 6–8 weeks of gestation, peaks at 32–34 weeks, and increases by 40–45%, with an average increase of approximately 1,450 ml. This elevated volume is maintained until delivery. Among this, plasma volume increases by an average of 1,000 ml, while red blood cell mass increases by approximately 450 ml. Since the increase in plasma volume exceeds the rise in red blood cell mass, physiological hemodilution occurs.
Blood Components
Red Blood Cells
Hematopoiesis in the bone marrow increases during pregnancy, and reticulocytes show a slight rise. Due to hemodilution, the red blood cell count is approximately (3.5–5.0) × 1012/L, hemoglobin levels range between 110–130 g/L, and hematocrit is around 0.31–0.34.
White Blood Cells
White blood cell counts demonstrate a slight increase during pregnancy, typically ranging between (5–12) × 109/L and occasionally reaching 15 × 109/L. During labor and the puerperium, white blood cell counts rise significantly, generally to (14–16) × 109/L, and in some cases, up to 25 × 109/L. This increase is mainly due to a rise in neutrophils, while lymphocytes show a less pronounced increase, and monocytes and eosinophils undergo minimal changes. White blood cell levels usually return to normal within 1–2 weeks postpartum.
Platelets
The changes in platelet counts during pregnancy remain uncertain. Factors such as increased platelet destruction, hemodilution, or immune mechanisms during pregnancy may lead to thrombocytopenia, and some pregnant individuals may develop gestational thrombocytopenia (GT) in late pregnancy. Although platelet counts may decrease, platelet function becomes enhanced to maintain hemostasis. Platelet counts typically return to normal within 1–2 weeks postpartum.
Coagulation Factors
The coagulation system shifts toward a hypercoagulable state during pregnancy, preparing the body to prevent excessive bleeding during the peripartum period. Coagulation factors II, V, VII, VIII, IX, and X increase, while factors XI and XIII decrease. During late pregnancy, the prothrombin time (PT) and activated partial thromboplastin time (APTT) shorten slightly, while clotting time shows no significant change. Plasma fibrinogen levels increase by approximately 50% compared to non-pregnant individuals, reaching an average of 4.5 g/L in late pregnancy (compared to 3 g/L in non-pregnant individuals). Venous stasis and vascular wall injury during pregnancy contribute to a hypercoagulable state, increasing the risk of thromboembolic events by 5–6 times compared to non-pregnant individuals. These physiological changes allow for the rapid formation of blood clots at the placental detachment site postpartum, serving as another important mechanism to prevent postpartum hemorrhage. Coagulation factor levels normalize within two weeks after delivery.
Plasma Proteins
Plasma protein levels decrease due to hemodilution, beginning in early pregnancy. By mid-pregnancy, plasma protein levels reach approximately 60–65 g/L, primarily due to a reduction in albumin, which decreases to around 35 g/L. This level is maintained until delivery.
Changes in the Urinary System
The kidneys enlarge slightly during pregnancy. Renal plasma flow (RPF) and glomerular filtration rate (GFR) increase during early pregnancy and remain elevated throughout gestation. Compared to the non-pregnant state, RPF increases by approximately 35%, and GFR rises by approximately 50%. This leads to increased excretion of metabolic waste products such as urea and creatinine, whose serum concentrations are lower than in the non-pregnant state. Both RPF and GFR are affected by body position, with urine output increasing in the supine position, resulting in greater nocturnal urine output compared to daytime. The rise in GFR during pregnancy, without a corresponding increase in tubular reabsorption of glucose, results in physiological glycosuria in approximately 15% of individuals after meals, necessitating differentiation from diabetes.
Compression of the urinary system by the enlarged uterus during pregnancy raises ureteral pressure. Progesterone further reduces the smooth muscle tone of the urinary tract. The ureters dilate and exhibit reduced peristalsis, causing slower urine flow. By mid-pregnancy, mild dilation of the renal pelvis and ureters is observed, with the right ureter more often affected due to the dextrorotation of the gravid uterus, potentially leading to hydronephrosis. Pregnant individuals are more prone to developing acute pyelonephritis, with the right side being more commonly affected. In early pregnancy, bladder compression by the enlarging uterus may cause increased frequency of urination, a symptom that often alleviates once the uterus ascends out of the pelvis. In late pregnancy, as the fetal head descends into the pelvis, increased pressure on the bladder and urethra may lead to frequency and occasional urinary incontinence.
Changes in the Respiratory System
During pregnancy, the costophrenic angle widens, and the ribs expand outward. The transverse and anteroposterior diameters of the thoracic cavity increase, leading to a greater circumference, while diaphragmatic elevation shortens the vertical diameter. However, the overall thoracic cavity volume remains unchanged, and vital capacity is not affected. Oxygen consumption increases by 10–20% by mid-pregnancy, and pulmonary ventilation rises by approximately 40%. Hyperventilation leads to an increase in arterial blood PO2 levels to 92 mmHg and a decrease in PCO2 levels to 32 mmHg. This facilitates the oxygen supply needed by the pregnant individual and the fetus while promoting the removal of fetal CO2 via the placenta. Respiratory rate remains mostly unchanged during pregnancy, staying below 20 breaths per minute, although respiration becomes deeper. Under the influence of estrogen, the mucosa of the upper respiratory tract (including the nasal passages, pharynx, and trachea) thickens, exhibits mild congestion and edema, and becomes more susceptible to upper respiratory infections.
Changes in the Digestive System
Under the influence of estrogen, the gums become hypertrophic during pregnancy, with a tendency toward congestion, edema, and bleeding. In some pregnant individuals, the gums develop localized vascular growths known as pregnancy epulis, which typically resolve naturally after childbirth. Progesterone causes relaxation of the lower esophageal sphincter, leading to reflux of acidic gastric contents into the lower esophagus and resulting in heartburn, although gastric emptying time remains unaffected. The liver does not enlarge, and liver function shows no significant changes. Gallbladder emptying time is prolonged, and bile becomes slightly more viscous, predisposing individuals to cholestasis, cholecystitis, and gallstone formation. Intestinal peristalsis slows, with prolonged stool retention in the large intestine leading to constipation. Increased rectal venous pressure further predisposes pregnant individuals to hemorrhoids or exacerbation of pre-existing hemorrhoids. The enlarging uterus causes upward and lateral displacement of the stomach and intestines, which may alter the typical clinical presentation of diseases. For example, appendicitis may present as mid- or upper-right abdominal pain.
Changes in the Endocrine System
Pituitary Gland
The pituitary gland enlarges during pregnancy, significantly so in late pregnancy, with marked hypertrophy of the anterior pituitary. Acidophilic cells proliferate and form "pregnancy cells."
- Gonadotropins (Gn): Large amounts of estrogen and progesterone secreted by the corpus luteum and placenta exert negative feedback on the hypothalamus and anterior pituitary, reducing the secretion of FSH and LH. As a result, ovarian follicles do not develop or mature, and ovulation ceases during pregnancy.
- Prolactin (PRL): Prolactin levels begin to increase at week 7 of pregnancy and continue to rise as pregnancy progresses, peaking at approximately 150 µg/L before term, which is about 10 times the level in non-pregnant individuals. Prolactin promotes mammary gland development, preparing the body for lactation after delivery.
Adrenal Cortex
During pregnancy, the secretion of adrenocorticotropic hormone (ACTH) increases, influenced by the high levels of estrogen. Cortisol secretion from the zona fasciculata of the adrenal cortex rises threefold, with approximately 75% of circulating cortisol bound to globulin, 15% bound to albumin, and only 10% in its active free form. Consequently, pregnant individuals do not exhibit symptoms of adrenal hyperfunction. Aldosterone secretion from the zona glomerulosa increases fourfold, but only 30–40% of circulating aldosterone is in its active free form, preventing excessive retention of sodium and water. Testosterone secretion from the innermost zona reticularis slightly increases, which results in the thickening or increased growth of pubic and axillary hair in some individuals.
Thyroid Gland
The thyroid gland shows moderate enlargement during pregnancy due to the influence of thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG). TSH levels transiently decrease during early pregnancy and return to pre-pregnancy levels by the end of the first trimester, remaining stable thereafter. Thyroxine-binding globulin (TBG) levels rise in early pregnancy, peaking at around 20 weeks, at approximately twice the pre-pregnancy levels, and then plateauing near this baseline. The increase in TBG results in higher total serum levels of thyroxine (T4) and triiodothyronine (T3), but free T4 and free T3 levels, which are physiologically active, remain unaffected. Total T4 begins to increase rapidly at 6–9 weeks of pregnancy, peaking at 18 weeks. Free T4 shows a slight increase, peaking along with hCG levels, and then returns to normal. A small amount of maternal T4 crosses the placenta to support fetal thyroid function. The fetal thyroid gland cannot concentrate iodine before 10–12 weeks of gestation. After 12 weeks, the fetal thyroid gland synthesizes and secretes thyroid hormones in response to TSH secreted by the fetal pituitary, but prior to this, the fetus depends on maternal thyroid hormones. At birth, approximately 30% of total T4 in umbilical cord blood originates from the mother. Neither maternal nor fetal TSH crosses the placenta, with each regulating their own thyroid function independently.
Parathyroid Glands
In early pregnancy, maternal serum levels of parathyroid hormone decrease. As blood volume and glomerular filtration rate increase, along with calcium transport to the fetus, maternal calcium concentrations gradually decline, causing parathyroid hormone levels to rise in mid-to-late pregnancy. This adaptation helps facilitate calcium supply to the fetus.
Changes in the Skin
During pregnancy, melanocyte-stimulating hormone (MSH) secretion increases. Additionally, estrogen and progesterone have melanocyte-stimulatory effects, leading to increased melanin production. This results in hyperpigmentation of areas such as the nipples, areolae, linea alba, and external genitalia. Pigmentation in the malar region may extend to areas such as the periorbital region, forehead, upper lip, and nose, forming a well-defined butterfly-patterned brownish discoloration known as chloasma gravidarum, which typically fades postpartum.
During pregnancy, increased glucocorticoid secretion by the adrenal cortex leads to the breakdown of elastin fibers in the skin, causing degeneration of elastic tissue. Along with increased tension on the abdominal skin due to uterine enlargement, these factors result in the formation of irregular, parallel, slightly depressed purple or pale red streaks, known as striae gravidarum (stretch marks). These marks are commonly seen in first-time pregnancies. In individuals with a history of pregnancy, older stretch marks may appear silvery and shiny.
Changes in Metabolism
Basal Metabolic Rate
The basal metabolic rate slightly decreases during early pregnancy, gradually increases during the second trimester, and reaches a 15–20% elevation by late pregnancy. The total additional energy requirement during pregnancy is approximately 80,000 kilocalories, which translates to an average increase of about 300 kilocalories per day (1 kcal ≈ 4.186 kJ).
Body Weight
The increase in body weight during pregnancy primarily comes from the uterus and its contents, the breasts, expanded blood volume, interstitial fluid, as well as small amounts of maternal fat and protein stores.
Carbohydrate Metabolism
During pregnancy, insulin secretion from the pancreas increases. However, placental production of insulinase and insulin-antagonistic hormones results in a relative insufficiency of insulin secretion. Fasting blood glucose levels are slightly lower in pregnant individuals, while postprandial hyperglycemia and hyperinsulinemia are observed, facilitating glucose supply to the fetus. These changes in glucose metabolism during pregnancy contribute to the development of gestational diabetes in some cases.
Fat Metabolism
Energy expenditure rises during pregnancy, leading to increased maternal fat storage and reduced glycogen reserves. In cases of excessive energy consumption, significant mobilization of fat occurs, resulting in elevated ketone body levels in the blood, which makes pregnant individuals more prone to ketosis.
Protein Metabolism
Protein requirements increase significantly during pregnancy, resulting in a positive nitrogen balance. Sufficient protein reserves are necessary not only for fetal growth and the enlargement of the uterus and breasts but also to prepare for the energy demands of labor. Insufficient protein reserves may lead to decreased plasma protein levels, increased interstitial fluid, and the onset of edema.
Mineral Metabolism
During pregnancy, total potassium and sodium stores increase, but, due to the rise in blood volume, serum potassium and sodium concentrations remain similar to those in the non-pregnant state. Serum phosphorus levels show no significant changes, while serum magnesium levels decrease. Fetal growth and development require substantial amounts of calcium, with the term fetus accumulating approximately 30 g of calcium, 80% of which is deposited during the final trimester. In light of this, dietary calcium intake should be enhanced during mid-to-late pregnancy, and calcium supplementation should be considered. Approximately 1,000 mg of iron is required during pregnancy, of which 300 mg is transported to the placenta and fetus, 500 mg is utilized for maternal red blood cell production, and 200 mg is lost through various physiological pathways (primarily gastrointestinal). Iron demand peaks during late pregnancy, averaging 6–7 mg per day. Since most pregnant individuals' iron stores are insufficient to meet these needs, additional iron supplementation may be necessary, if indicated, to support fetal development and maternal requirements.
Changes in Bones, Joints, and Ligaments
Bone density generally remains unchanged during pregnancy, except in cases of frequent and closely spaced pregnancies combined with inadequate vitamin D and calcium supplementation, which may lead to osteoporosis. Some pregnant individuals report discomfort or pain in the lumbosacral region and limbs, which may be related to relaxin secretion by the placenta. Relaxin contributes to the relaxation of the pelvic ligaments as well as the joints and ligaments between vertebrae. In some cases, relaxation and separation of the pubic symphysis result in significant pain and restricted movement, although these symptoms often resolve postpartum.