Fetal adnexa include the placenta, fetal membranes, umbilical cord, and amniotic fluid, which play crucial roles in maintaining the life, growth, and development of the fetus.
Placenta
Structure of the Placenta
The placenta is composed of the fetal part, which includes the amnion and chorion frondosum, and the maternal part, which consists of the decidua basalis.
Amnion
The amnion is a transparent thin membrane attached to the fetal surface of the placenta. It has a smooth surface and is devoid of blood vessels, nerves, and lymphatic tissues. The normal thickness of the amnion ranges from 0.02 to 0.05 mm. Under electron microscopy, microvilli are present on the surface of amniotic epithelial cells, allowing for the exchange of substances between amniotic fluid and the amnion.
Chorion Frondosum
The chorion frondosum forms the main structure of the placenta. After implantation of the blastocyst, trophoblast cells proliferate rapidly at the site of implantation, forming two layers: the inner layer of cytotrophoblasts (characterized by proliferative growth) and the outer layer of syncytiotrophoblasts (responsible for functional activities and derived from cytotrophoblast differentiation). On the inner side of the trophoblast layer, there is a layer of extraembryonic mesoderm that, together with the trophoblast layer, constitutes the chorion. The chorion in contact with the decidua basalis becomes enriched with nutrients and well-developed, forming the chorion frondosum. Its formation involves three stages:
- Primary Villi: Cytotrophoblast columns arranged radially grow from the surface of the chorion, and actively proliferating cytotrophoblasts extend into the core, forming cellular cords within the syncytiotrophoblast columns.
- Secondary Villi: The primary villi elongate, and the core cellular cords are infiltrated by the extraembryonic mesoderm, forming mesenchymal cores.
- Tertiary Villi: On days 15 to 17 post-fertilization, embryonic blood vessels invade the mesenchymal cores, leading to the formation of vascularized villi.
A primary villous trunk, along with its branches, forms a fetal cotyledon, while a secondary villous trunk and its branches form a fetal lobule. Each placenta contains 60–80 fetal cotyledons and approximately 200 fetal lobules. Within each villous trunk and its branches, branches of the umbilical artery and umbilical vein progressively narrow and extend, eventually forming fetal capillaries in the tertiary villi, establishing the fetal-placental circulation. The spaces between the villi are known as intervillous spaces (IVS). During trophoblast invasion of the uterine wall, uterine spiral arteries rupture and open directly into the intervillous spaces, where maternal blood fills these spaces. Free-floating villi remain suspended in maternal blood, facilitating the exchange of substances between the fetus and mother.
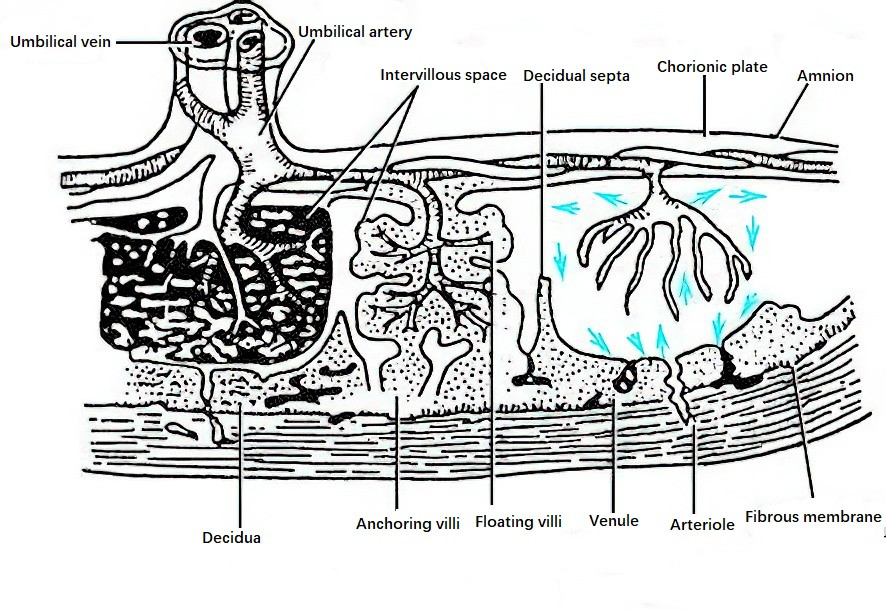
Figure 1 Diagram of placental structure and the fetal-placental circulation
The establishment of the uterine-placental circulation involves remodeling of the uterine spiral arteries, a process carried out by two types of extravillous trophoblasts:
- Interstitial Trophoblasts: These penetrate the decidua, endometrium, and the inner one-third of the myometrium, gathering around the spiral arteries and preparing for the invasion of intravascular trophoblasts.
- Intravascular Trophoblasts: These migrate retrogradely along the lumina of the uterine spiral arteries, replacing endothelial cells and transforming the narrow muscular channels into dilated, low-resistance uteroplacental vessels.
In early pregnancy, intravascular trophoblasts at the ends of the spiral arteries form emboli, temporarily blocking these vessels. At the end of early pregnancy, the emboli dissolve, allowing the uterine-placental circulation to be established. Impaired spiral artery remodeling may result in preeclampsia, fetal growth restriction (FGR), or both. In cases of severe preeclampsia with FGR, only 10% of the uterine spiral arteries undergo complete remodeling, compared to a remodeling rate of 96% in normal pregnancies.
At term, the placental villi cover a surface area of 12–14 m2, comparable to the total surface area of an adult’s intestine, providing a vast area for maternal-fetal exchange. Oxygen-depleted fetal blood with high concentrations of metabolic waste products flows through the umbilical arteries into the villous capillaries. Nutrients and oxygen are exchanged with maternal blood in the intervillous spaces, and oxygen-rich, nutrient-laden blood returns to the fetus via umbilical veins, ensuring fetal growth and development. Fetal and maternal blood do not mix directly; they are separated by the capillary wall of the villi, the villous stroma, and the trophoblast layer of the villi. This structure forms the maternal-fetal interface, which serves as the placental barrier and has roles in immune tolerance.
Decidua Basalis
This structure constitutes the endometrium at the site of placental attachment and represents a minor portion of the placenta. Cytotrophoblasts anchoring the villi, together with the decidua basalis, form the base of the intervillous spaces, known as the decidual plate. Decidual septa extend from the decidual plate into the chorionic surface, dividing the maternal side of the placenta into approximately 20 lobules visible to the naked eye. The extensions do not exceed two-thirds of the placental thickness.
At term, the placenta is disk-shaped, typically circular or oval, weighing 450–650 g, with a diameter of 16–20 cm and a thickness of 1–3 cm. The central portion is about 3 cm thick, gradually thinning towards the edges. The placenta consists of a fetal side and a maternal side. The fetal side is covered with amnion, appearing grayish-white, smooth, and semi-transparent. Branches of the umbilical arteries and veins radiate outward from their attachment point toward the periphery, traversing the chorionic plate and entering the villous trunks and branches. The maternal side is dark red, with decidual septa forming shallow grooves that divide the surface into several maternal lobules.
Functions of the Placenta
The placenta, positioned between the fetus and the mother, functions as a critical organ to sustain fetal growth and development. It performs roles in material exchange, defense, synthesis, and immunity.
Material Exchange
The placenta facilitates material exchange through various mechanisms, including gas exchange, nutrient supply, and the elimination of fetal metabolic waste products.
Gas Exchange
The exchange of oxygen (O2) and carbon dioxide (CO2) between the mother and fetus occurs in the placenta via simple diffusion, fulfilling a role equivalent to the fetal respiratory system. The partial pressure of oxygen (PO2) in uterine arterial blood is higher than that in the blood of the intervillous spaces and fetal umbilical arterial blood, but fetal hemoglobin has a higher affinity for oxygen, ensuring sufficient uptake of O2 from maternal blood. Conversely, CO2 diffuses 20 times faster than O2, and fetal blood's affinity for CO2 is lower than maternal blood's, allowing CO2 from the fetus to rapidly diffuse through the intervillous spaces to the maternal side.
Nutrient Supply
Glucose, the primary energy source for fetal metabolism, is transported across the placenta via facilitated diffusion, and all glucose in the fetal body originates from the mother. Amino acids, calcium, phosphorus, iodine, and iron are transferred through active transport mechanisms, while free fatty acids, water, potassium, sodium, magnesium, and vitamins A, D, E, and K cross the placenta via simple diffusion.
Elimination of Fetal Metabolic Waste
Metabolic waste products from the fetus, such as urea, uric acid, creatinine, and creatine, are transferred across the placenta into maternal blood, where they are subsequently excreted by the maternal body.
Defense Function
The placental barrier offers limited protective functionality. Various viruses, including rubella virus and cytomegalovirus, as well as most drugs, can cross the placenta, potentially affecting fetal growth and development. Bacteria, Toxoplasma, Chlamydia, and Treponema pallidum cannot penetrate the placental barrier directly but may form lesions in the placenta, disrupting villous structures and infecting the embryo or fetus. Maternal immune antibodies, such as IgG, can cross the placenta, providing the fetus with passive immunity for a short period after birth.
Synthetic Function
Syncytiotrophoblasts in the placenta are capable of synthesizing various hormones, enzymes, neurotransmitters, and cytokines, which play a vital role in maintaining a normal pregnancy.
Human Chorionic Gonadotropin (hCG)
hCG is a glycoprotein hormone composed of alpha (α) and beta (β) subunits. It can be detected in maternal serum as early as one day after implantation and peaks at 8–10 weeks of pregnancy, declining rapidly afterward and disappearing within two weeks postpartum. The functions of hCG include:
- Supporting the lifespan of the corpus luteum by transforming it into the corpus luteum of pregnancy, which increases the secretion of steroid hormones needed to sustain pregnancy.
- Promoting the aromatization of androgens into estrogens and stimulating progesterone synthesis.
- Suppressing the activation of lymphocytes by phytohemagglutinin and adhering to the trophoblast surface to protect it from maternal lymphocyte-mediated attack.
- Stimulating testosterone secretion by the fetal testes to facilitate male fetal sexual differentiation.
- Binding to thyroid-stimulating hormone (TSH) receptors on maternal thyroid cells, thereby stimulating thyroid activity.
Human Placental Lactogen (hPL)
hPL is a single-chain polypeptide hormone detectable in maternal plasma by the fifth week of pregnancy. Its secretion increases progressively with gestation, reaching a peak in late pregnancy and remaining stable until delivery, after which levels drop rapidly and become undetectable within seven hours postpartum. The functions of hPL include:
- Promoting the development of mammary gland alveoli and stimulating epithelial cells to synthesize casein, lactalbumin, and beta-lactoglobulin in preparation for lactation.
- Enhancing insulin production.
- Increasing free fatty acid and glycerol concentrations through lipolysis, utilizing free fatty acids as an energy source, reducing maternal glucose uptake, and directing glucose to the fetus as a primary energy and protein-synthesis substrate.
- Reducing maternal immune rejection of the fetus.
hPL is recognized as a "metabolic modulator" that supports fetal development through maternal adaptations.
Estrogen
Estrogen is a steroid hormone initially secreted by the ovarian corpus luteum in early pregnancy and primarily synthesized by the fetoplacental unit after 10 weeks of gestation. By late pregnancy, estriol levels reach 1,000 times those of nonpregnant women, while estradiol and estrone levels increase to 100 times their nonpregnant values. The synthesis of estrogen involves several steps:
- Maternal cholesterol is converted into pregnenolone in the placenta.
- Pregnenolone is transformed into dehydroepiandrosterone sulfate (DHEAS) by the fetal adrenal cortex.
- DHEAS undergoes 16α-hydroxylation in the fetal liver, forming 16α-hydroxy-DHEAS.
- Placental sulfatases remove sulfate groups from 16α-hydroxy-DHEAS, forming 16α-hydroxy-DHEA.
- Finally, placental aromatase converts 16α-hydroxy-DHEA into free estriol.
Progesterone
Progesterone is a steroid hormone produced by the corpus luteum of pregnancy during early gestation. After 8–10 weeks of pregnancy, the syncytiotrophoblasts of the placenta become the primary source of progesterone. Maternal progesterone levels increase progressively throughout pregnancy, with its major metabolite being pregnanediol. Progesterone, in collaboration with estrogen, plays an important role in inducing physiological changes in the endometrium, myometrium, mammary glands, and other systems during pregnancy.
Oxytocinase
Oxytocinase is a glycoprotein that increases gradually as pregnancy progresses, reaching a peak during late pregnancy. Its biological significance is not fully understood, though it primarily functions to inactivate oxytocin molecules, thereby maintaining pregnancy. In cases of placental dysfunction, such as stillbirth, preeclampsia, or fetal growth restriction (FGR), oxytocinase levels in the blood are reduced.
Heat Stable Alkaline Phosphatase (HSAP)
HSAP becomes detectable in maternal blood between 16 and 20 weeks of gestation. Its levels increase progressively with pregnancy and decrease following delivery of the placenta, disappearing entirely 3–6 days postpartum. Monitoring changes in HSAP levels can provide an indicator for evaluating placental function.
Cytokines and Growth Factors
Factors such as epidermal growth factor (EGF), nerve growth factor, insulin-like growth factor (IGF), tumor necrosis factor-α (TNF-α), and interleukins (IL-1, IL-2, IL-6, IL-8, etc.) contribute to fetal nutrition and immune protection.
Immune Function
The fetus represents a semi-allogeneic graft to the maternal body. Despite this, normal pregnancies exhibit maternal tolerance without rejection of the fetus. The precise mechanisms are not fully understood, though they may involve the lack of antigenicity in early embryonic tissues, immune tolerance at the maternal-fetal interface, and reduced maternal immunity during pregnancy.
Fetal Membranes
The fetal membranes consist of the outer chorion laeve and the inner amnion. During development, the non-implantation regions of the chorion degenerate and atrophy due to insufficient nutrition, forming the chorion laeve (previously referred to as the smooth chorion). The fetal membranes play a critical role in maintaining the integrity of the amniotic cavity, providing protection to the fetus. The membranes contain phospholipids rich in arachidonic acid (a precursor of prostaglandins) and lysosomal enzymes capable of catalyzing the release of free arachidonic acid from phospholipids, contributing to the initiation of labor.
Umbilical Cord
The umbilical cord is a cord-like structure that connects the fetus to the placenta, enabling the fetus to remain suspended in amniotic fluid. At full term, the umbilical cord measures 30–100 cm in length, with an average length of approximately 55 cm and a diameter of 0.8–2.0 cm. It appears helically coiled and is covered with a grayish-white amniotic membrane. It contains one umbilical vein and two umbilical arteries, surrounded by a gelatinous connective tissue derived from the extraembryonic mesoderm called Wharton’s jelly, which serves to protect the umbilical vessels. The umbilical cord is a vital passageway for gas exchange, nutrient delivery, and the elimination of metabolic waste between the mother and fetus. Compression of the umbilical cord may impede blood flow, resulting in fetal hypoxia and even posing a threat to fetal life.
Amniotic Fluid
The fluid that fills the amniotic cavity is referred to as amniotic fluid.
Sources of Amniotic Fluid
In early pregnancy, amniotic fluid primarily originates from maternal serum, which enters the amniotic cavity via diffusion through the fetal membranes.
After mid-pregnancy, fetal urine becomes the main source of amniotic fluid, leading to a gradual reduction in its osmotic pressure.
In late pregnancy, the fetal lungs contribute to the production of amniotic fluid, releasing approximately 350 ml of fluid daily into the amniotic cavity.
Small amounts of fluid also originate from the amnion, Wharton’s jelly of the umbilical cord, and exudates from fetal skin.
Absorption of Amniotic Fluid
The primary mechanism for the absorption of amniotic fluid is fetal swallowing. Fetal swallowing begins around 10–12 weeks of gestation, and near term, the fetus can swallow 500–700 ml of fluid daily. Due to the hypotonic nature of amniotic fluid compared to maternal plasma, another important route of absorption involves intramembranous transport across the amniochorionic interface into fetal placental blood vessels. Only a small amount of amniotic fluid transfers into maternal plasma. Intramembranous transport may work synergistically with fetal swallowing to stabilize amniotic fluid volume. Additionally, the umbilical cord can absorb 40–50 ml of amniotic fluid per hour. Before 20 weeks of gestation, the fetal pre-keratinized skin also has the capacity to absorb amniotic fluid, but the amount is minimal.
Fluid Balance Between the Mother, Fetus, and Amniotic Fluid
A continuous exchange of fluid occurs within the amniotic cavity to maintain a relatively stable amniotic fluid volume. The exchange of fluid between the mother and fetus happens primarily through the placenta, at a rate of approximately 3,600 ml per hour. Regulation of amniotic fluid volume depends on the following four factors:
- Starting in the second half of pregnancy, fetal urination becomes the primary source of amniotic fluid.
- The secretion of alveolar fluid by the fetal lungs.
- Approximately 400 ml of amniotic fluid transported daily via intramembranous pathways into fetal blood vessels on the placental surface.
- Fetal swallowing serves as the main pathway for amniotic fluid absorption.
Volume, Characteristics, and Composition of Amniotic Fluid
The volume of amniotic fluid gradually increases during pregnancy, reaching approximately 1,000 ml at 38 weeks of gestation. After this point, its volume decreases gradually, averaging about 800 ml at 40 weeks of gestation. In cases of post-term pregnancy, the amniotic fluid volume decreases significantly and may drop below 300 ml.
Early in pregnancy, amniotic fluid appears as a colorless, clear fluid. At term, it becomes slightly turbid and opaque, with small suspended particles (vernix caseosa, desquamated fetal epithelial cells, lanugo, hair, a small number of leukocytes, albumin, urate crystals, etc.). Amniotic fluid contains numerous hormones and enzymes. At term, its specific gravity ranges from 1.007 to 1.025, with a pH of approximately 7.20. The fluid consists of 98%–99% water, with the remaining 1%–2% comprising inorganic salts and organic substances.
Functions of Amniotic Fluid
Protection of the Fetus
The amniotic cavity maintains a constant temperature, and an appropriate amount of amniotic fluid provides a cushioning effect to protect the fetus from external pressure. It prevents adhesion of fetal limbs, reduces direct compression of the umbilical cord by the uterine muscle wall or fetus, and minimizes the risk of fetal distress. During labor, amniotic fluid helps to evenly distribute the pressure of uterine contractions, reducing the likelihood of localized compression-induced fetal distress. Fetal swallowing or inhalation of amniotic fluid promotes the development of the digestive tract and lungs. Insufficient amniotic fluid volume can result in fetal lung hypoplasia.
Protection of the Mother
Amniotic fluid mitigates discomfort caused by fetal movements. During labor, the bulging amniotic sac exerts wedge-shaped hydraulic pressure to dilate the cervix and vagina. After membrane rupture, the outflow of amniotic fluid flushes the vaginal canal, reducing the risk of infection.