The regulation of the menstrual cycle is a highly complex process primarily involving the hypothalamus, pituitary gland, and ovaries. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which regulates the secretion of gonadotropins from the pituitary and, in turn, modulates ovarian function. Hormones secreted by the ovaries not only act on the endometrium but also exert feedback regulatory effects on the hypothalamus and pituitary. The interactions and mutual regulation among the hypothalamus, pituitary, and ovaries constitute an integrated and coordinated neuroendocrine system known as the hypothalamic-pituitary-ovarian (HPO) axis. In addition to the interactions among hypothalamus, pituitary, and ovarian hormones, the inhibin-activin-follistatin system also participates in the regulation of the menstrual cycle. The neuroendocrine activity of the HPO axis is influenced by higher brain centers, while the functions of other endocrine glands are also related to the menstrual cycle.
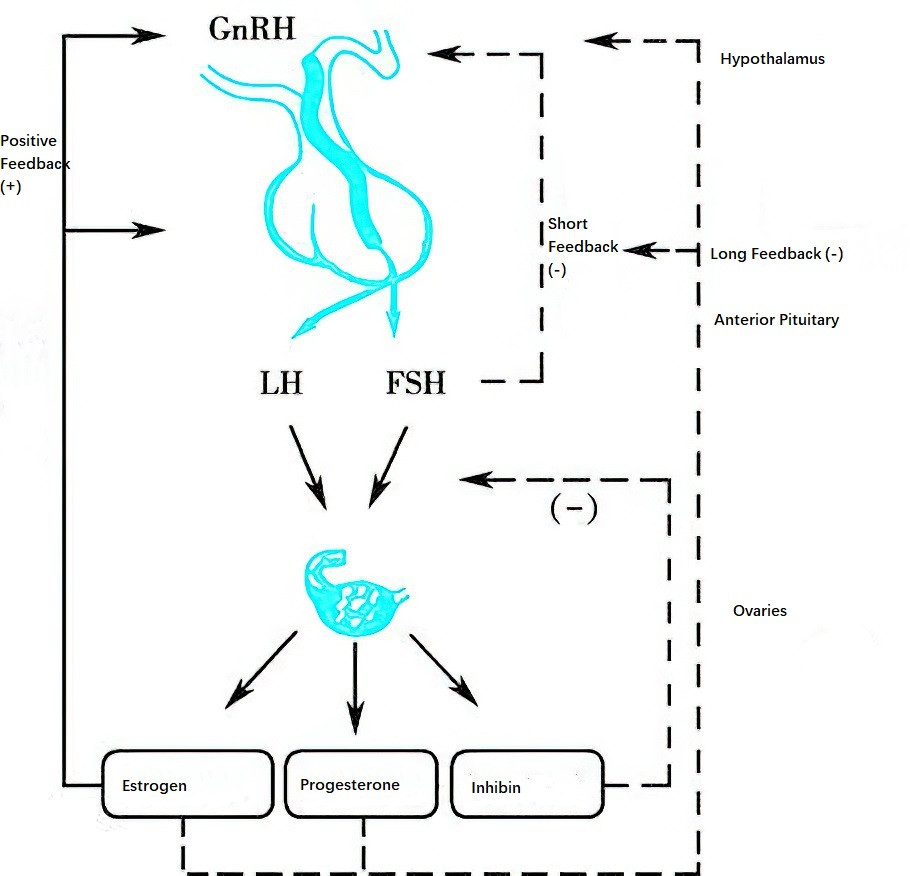
Figure 1 Mutual regulation among the hypothalamic-pituitary-ovarian (HPO) axis during the menstrual cycle
Hypothalamic Gonadotropin-Releasing Hormone
GnRH, a decapeptide hormone secreted by neurons in the arcuate nucleus of the hypothalamus, is delivered directly to the anterior pituitary through the pituitary portal system, where it regulates the synthesis and secretion of gonadotropins. GnRH is released in a pulsatile manner, with pulse intervals ranging between 60 and 120 minutes. The pulse frequency varies depending on the phase of the menstrual cycle. Both physiological functions and pathological changes associated with the normal menstrual cycle are accompanied by corresponding changes in the pulsatile secretion pattern of GnRH. The pulsatile release of GnRH regulates the ratio of luteinizing hormone (LH) to follicle-stimulating hormone (FSH). When the pulse frequency decreases, blood levels of FSH increase while LH levels decrease, resulting in a lower LH/FSH ratio. Conversely, an increased pulse frequency results in a higher LH/FSH ratio.
The hypothalamus serves as the initiating center of the HPO axis, and GnRH secretion is subject to feedback regulation by ovarian sex hormones and pituitary gonadotropins. This feedback regulation includes both positive feedback, which promotes GnRH secretion, and negative feedback, which inhibits it. Feedback mechanisms can be classified into long feedback, short feedback, and ultrashort feedback. Long feedback refers to the feedback effects of ovarian sex hormones circulating in the bloodstream on the hypothalamus. Short feedback involves inhibitory feedback from pituitary hormones on hypothalamic GnRH secretion. Ultrashort feedback refers to GnRH's negative feedback on its own synthesis.
In addition to hormonal feedback signals, neuroendocrine signals from higher brain centers regulate GnRH secretion through various neurotransmitters, such as norepinephrine, dopamine, beta-endorphin, serotonin, and melatonin. Norepinephrine promotes GnRH release, while beta-endorphin and serotonin inhibit its release. Dopamine exhibits dual effects on GnRH release, being capable of both promoting and inhibiting it.
Gonadotropins from the Anterior Pituitary
The anterior pituitary (adenohypophysis) secretes hormones that are directly involved in reproductive regulation, including gonadotropins and prolactin.
Gonadotropins
Gonadotropin-secreting cells in the anterior pituitary produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Both hormones are secreted in a pulsatile pattern in response to the pulsatile stimulation by GnRH, and their secretion is further regulated by ovarian hormones and inhibin. FSH and LH are both glycoprotein hormones composed of two polypeptide subunits, alpha and beta, which are covalently linked. The alpha subunit is identical in structure for all glycoprotein hormones, while the beta subunit differs and determines each hormone's specific antigenicity and biological activity. However, both subunits must combine to form a complete molecule for the hormone to exhibit biological activity. The structure of the alpha subunit is highly conserved among different glycoprotein hormones, including thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG), while the specificity of these hormones lies in their beta subunits.
The physiological roles of FSH include:
- Stimulating the proliferation and differentiation of granulosa cells in preantral and antral follicles, promoting the secretion of follicular fluid, and facilitating follicular development and growth.
- Activating aromatase in granulosa cells, thereby stimulating the synthesis and secretion of estradiol.
- Recruiting a cohort of antral follicles during the late luteal phase of the previous cycle and early follicular phase of the current cycle.
- Inducing granulosa cells to synthesize and secrete various substances such as insulin-like growth factor (IGF), its receptors, inhibin, and activin, while acting in synergy with these factors to regulate the selection of the dominant follicle versus the degeneration of non-dominant follicles.
- Acting in conjunction with estrogen in the late follicular phase to induce the production of LH receptors in granulosa cells, thereby preparing for ovulation and luteinization.
The physiological roles of LH include:
- Stimulating the synthesis of androgens, primarily androstenedione, in thecal cells during the follicular phase to serve as substrates for estradiol synthesis.
- Promoting final oocyte maturation and ovulation prior to ovulation.
- Sustaining luteal function during the luteal phase by enhancing the synthesis and secretion of progesterone, estradiol, and inhibin A.
Prolactin (PRL)
Prolactin (PRL) is a polypeptide hormone composed of 198 amino acids that is secreted by lactotroph cells in the anterior pituitary. It promotes the synthesis of breast milk. Its secretion is primarily regulated by inhibitory effects of dopamine (PRL-inhibiting factor) released into the portal circulation by the hypothalamus. Thyrotropin-releasing hormone (TRH) can also stimulate PRL secretion. Since dopamine and GnRH often exhibit simultaneous effects on stimulation or inhibition, suppression of GnRH secretion can lead to decreased gonadotropin levels and increased PRL levels, which may result in clinical manifestations such as the syndrome of amenorrhea-galactorrhea. Additionally, elevated TRH may cause some women with hypothyroidism to experience lactation.
Feedback Effects of Ovarian Hormones
Sex hormones secreted by the ovaries, including estrogen and progesterone, exert feedback regulatory effects on the hypothalamus and pituitary.
Estrogen
Estrogen exerts both negative and positive feedback effects on the hypothalamus. During the early follicular phase, a certain level of estrogen exerts negative feedback on the hypothalamus, inhibiting GnRH release and reducing the pituitary's responsiveness to GnRH, thereby suppressing the pulsatile secretion of gonadotropins by the pituitary. During the late follicular phase, as follicles develop and mature, estrogen secretion reaches a threshold (≥200 pg/ml) and sustains this level for more than 48 hours, at which point it exerts positive feedback effects, stimulating an LH surge. During the luteal phase, estrogen cooperates with progesterone to exert negative feedback effects on the hypothalamus.
Progesterone
Before ovulation, low levels of progesterone enhance the positive feedback effects of estrogen on gonadotropins. During the luteal phase, high levels of progesterone inhibit the pulsatile secretion of gonadotropins through negative feedback mechanisms.
Mechanisms Regulating the Menstrual Cycle
Follicular Phase
After the regression of the corpus luteum in the previous menstrual cycle, levels of estrogen, progesterone, and inhibin A reach their lowest points, lifting the inhibition on the hypothalamus and pituitary. The hypothalamus resumes GnRH secretion, leading to increased FSH secretion by the pituitary. This promotes follicular development and estrogen secretion, initiating the proliferative phase of the endometrium. As estrogen levels gradually rise, negative feedback on the hypothalamus intensifies, suppressing GnRH secretion. Additionally, inhibin B contributes to the suppression of FSH secretion by the pituitary. As the dominant follicle approaches full maturity, estrogen levels exceed 200 pg/ml and remain elevated for over 48 hours, exerting positive feedback effects on the hypothalamus and pituitary. This results in LH and FSH surges, which together trigger ovulation of the mature follicle.
Luteal Phase
Following ovulation, levels of circulating LH and FSH decline sharply. Under the influence of small amounts of LH and FSH, the corpus luteum forms and undergoes maturation. The corpus luteum predominantly secretes progesterone, along with estradiol, leading to the transformation of the endometrium into the secretory phase. Progesterone levels peak 7–8 days after ovulation, accompanied by another peak in estrogen. Together with inhibin A, the high levels of progesterone and estrogen exert negative feedback on the pituitary, suppressing LH and FSH secretion. If the released ovum is not fertilized, the corpus luteum begins to regress, leading to a decline in estrogen and progesterone secretion. In the absence of hormonal support, the endometrium undergoes desquamation, resulting in menstruation. The decrease in estrogen, progesterone, and inhibin A alleviates the negative feedback on the hypothalamus and pituitary, allowing FSH secretion to rise and stimulating the development of new follicles, marking the start of a new menstrual cycle. This process repeats cyclically.
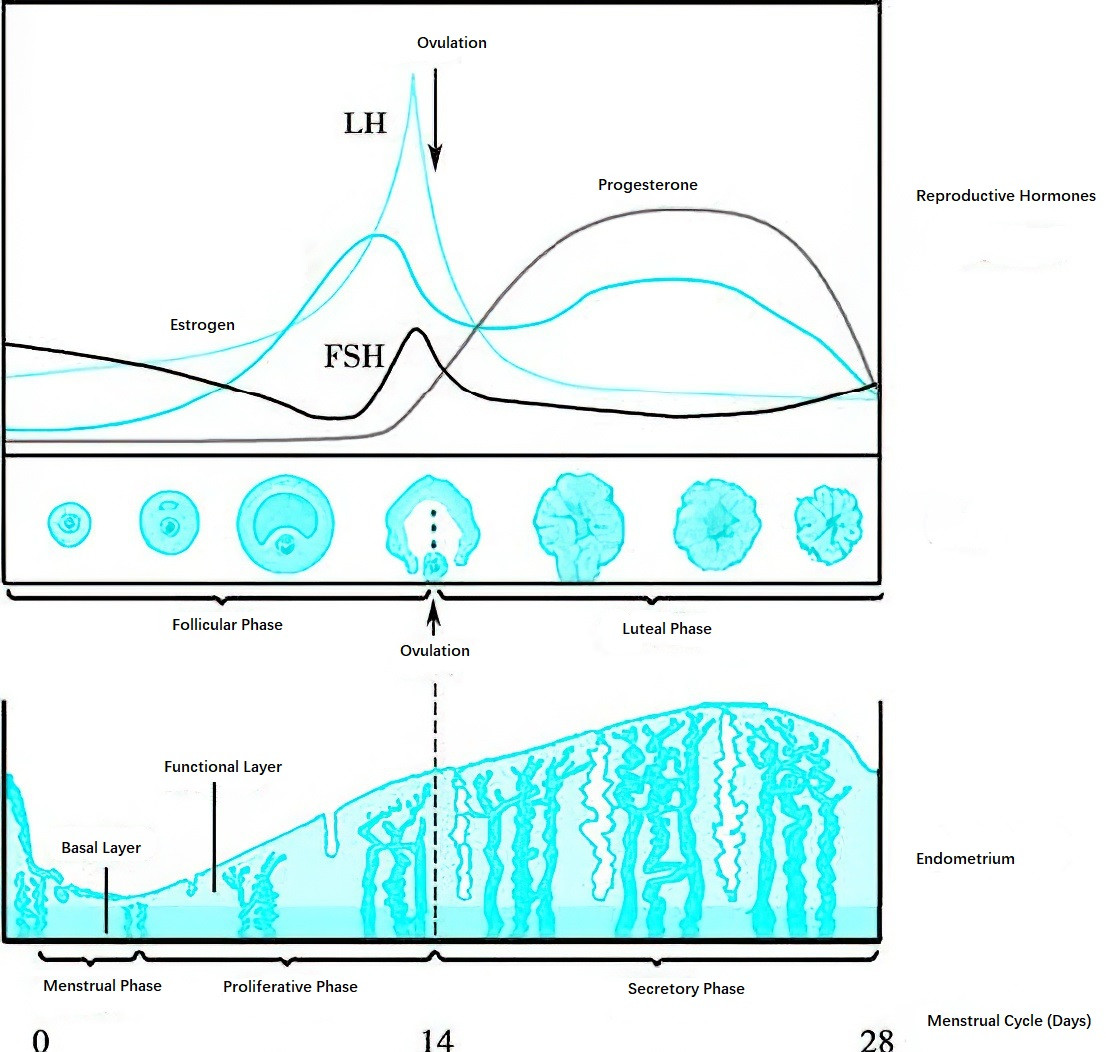
Figure 2 Diagram of cyclic changes in the ovary and endometrium and their relationship with hormone levels
The menstrual cycle is primarily regulated by the neuroendocrine activity of the HPO axis, with additional involvement from the inhibin-activin-follistatin system. Hormones from other endocrine glands also influence the menstrual cycle. The physiological activity of the HPO axis is affected by the brain's cortical neurological centers, and external factors such as environmental changes and psychological stress can also impact the menstrual cycle. Any disruption at any level of the cortical, hypothalamic, pituitary, or ovarian systems may result in ovarian dysfunction and menstrual irregularities.