Ovarian function undergoes significant changes across different stages of a woman’s life.
Ovarian Function
The ovary, the female gonad, serves two primary functions: the production and release of oocytes (ovulation) and the secretion of female hormones. These functions are respectively referred to as the reproductive and endocrine roles of the ovary.
Cyclical Changes in the Ovary
At 6–8 weeks of embryonic development, primordial germ cells undergo mitosis, leading to an increase in both cell number and size, forming oogonia (approximately 600,000 cells). From 11–12 weeks of development, oogonia begin the first meiotic division, becoming arrested in the prophase-diplotene stage, at which point they are called primary oocytes. By 16–20 weeks of gestation, the number of germ cells peaks at approximately 6–7 million across both ovaries, with one-third consisting of oogonia and two-thirds consisting of primary oocytes. Between the 16th week of gestation and six months after birth, spindle-shaped pre-granulosa cells derived from cortical cells of the gonadal sex cords form a single layer around the primary oocytes, resulting in the formation of primordial follicles, also known as primary follicles. This is the basic reproductive unit and the only form of reserve for female oocytes.
Following their formation, follicles enter a trajectory of autonomous development and atresia, a process that is not reliant on gonadotropins, though the underlying mechanism remains unclear. During fetal development, follicles continuously undergo atresia, leaving approximately 2 million remaining at birth. This number decreases further during childhood due to follicular degeneration, resulting in about 300,000 follicles by the onset of puberty.
From puberty until menopause, the ovaries undergo cyclical changes in both morphology and function, a process referred to as the ovarian cycle.
Follicular Development and Maturation
After puberty begins, follicular development transitions from autonomous growth to hormonally stimulated maturation, driven by gonadotropins. During the reproductive years, a cohort of follicles develops each month, with one typically maturing into a dominant follicle capable of ovulating an oocyte, while the others undergo programmed cell death (apoptosis) in a process called follicular atresia. Across a woman’s lifetime, approximately 400–500 follicles mature and ovulate, comprising only about 0.1% of the total initial follicle pool.
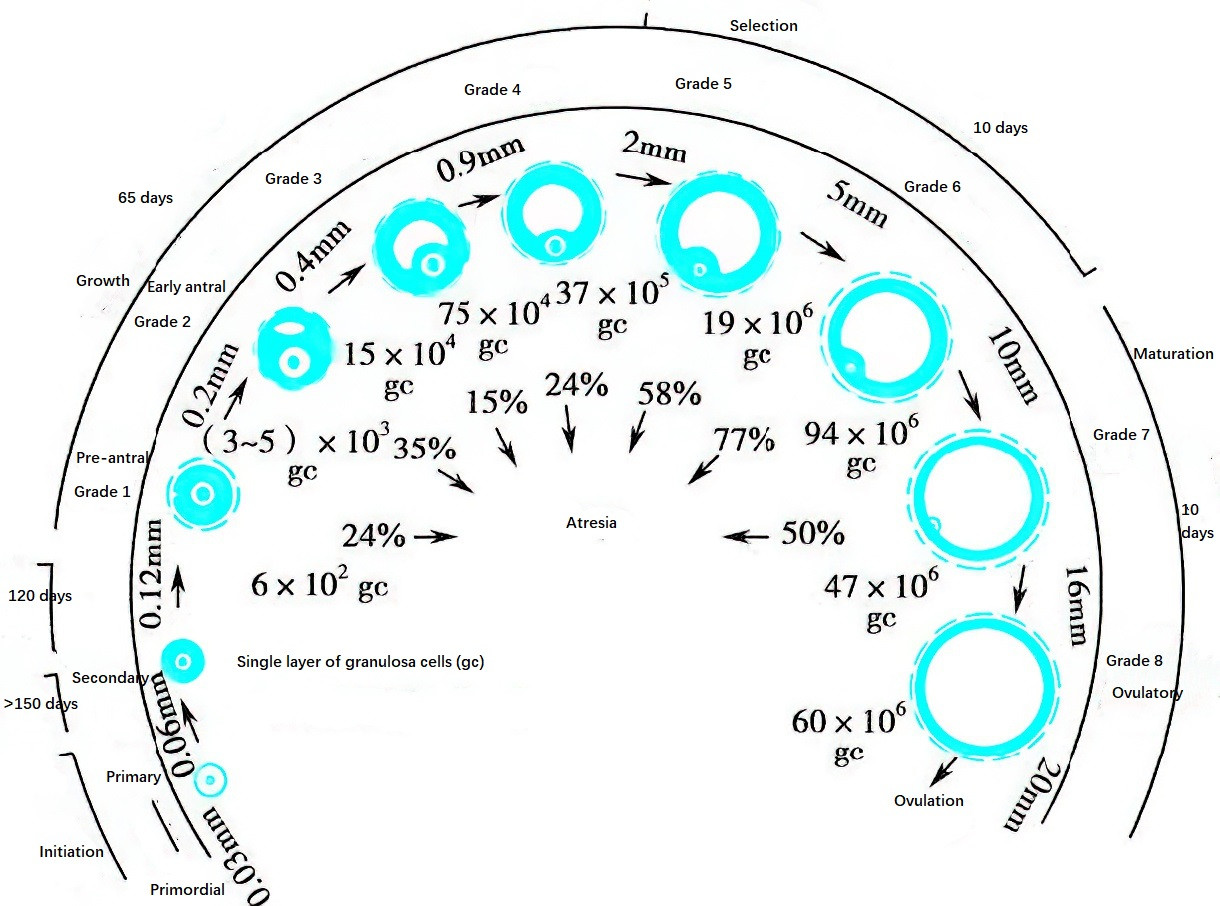
Figure 1 Growth and development of follicles in the adult ovary and proportions of follicles at different growth stages
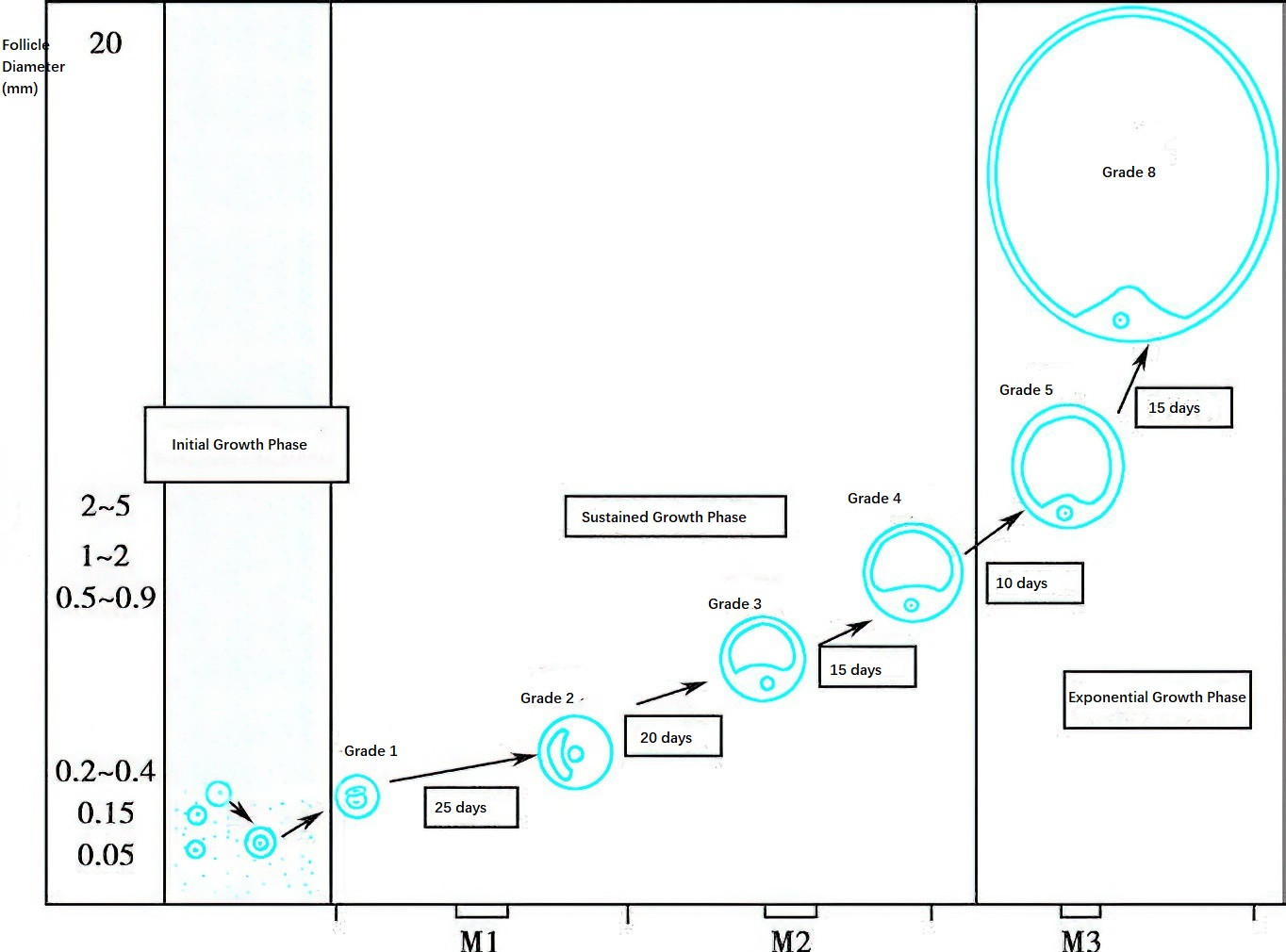
Figure 2 Diagram of follicular growth rate
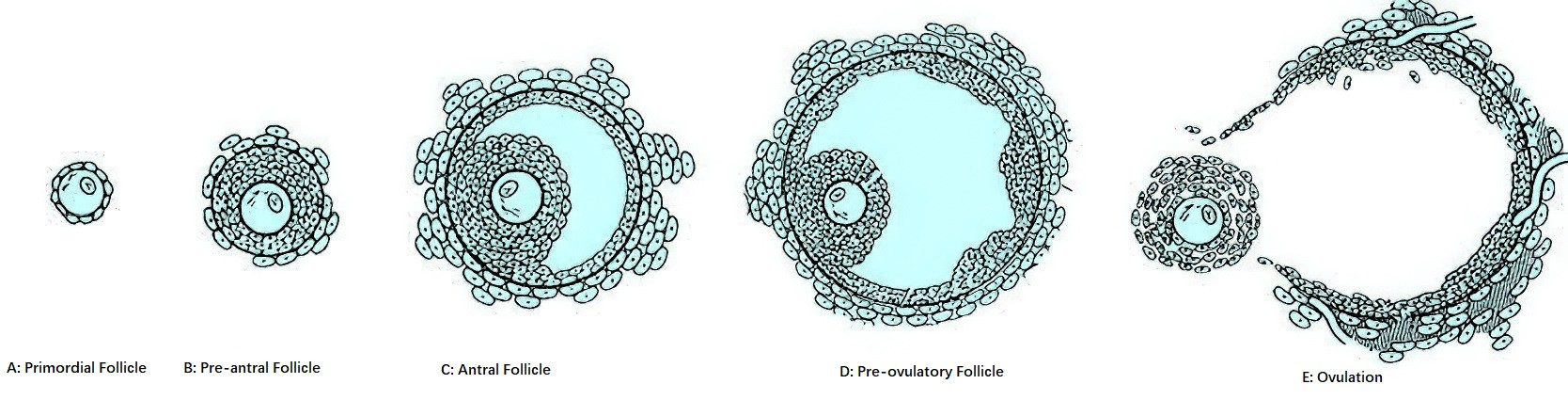
Figure 3 Diagram of follicle morphology at different developmental stages
Follicular development begins with the transformation of primordial follicles into primary follicles. Primordial follicles can remain dormant in the ovaries for decades. Follicular development begins months prior to the onset of the menstrual cycle. Transitioning from primordial follicles to pre-antral follicles takes more than nine months, and the maturation of pre-antral follicles into mature follicles spans about 85 days, crossing approximately three menstrual cycles. The final stages of follicular growth normally require 15 days and correspond to the follicular phase of the menstrual cycle.
The growth of follicles, based on their morphology, size, growth rate, and histological characteristics, can be categorized into the following stages:
Primordial Follicle
The primordial follicle consists of a primary oocyte arrested in the diplotene stage of prophase I, surrounded by a single layer of spindle-shaped pre-granulosa cells.
Pre-Antral Follicle
The pre-antral follicle includes primary and secondary follicles. A primordial follicle becomes a primary follicle when its spindle-shaped pre-granulosa cells differentiate into a single layer of cuboidal granulosa cells. Simultaneously, the granulosa cells synthesize and secrete mucopolysaccharides, forming a transparent ring-like zone around the oocyte, referred to as the zona pellucida. The cell membranes of the granulosa cells extend into the zona pellucida to establish gap junctions with the oocyte’s membrane, creating channels for nutrient transfer and communication. Subsequent proliferation of granulosa cells results in 6–8 cell layers (fewer than 600 cells), enlarging the follicle and forming a secondary follicle. Within secondary follicles, granulosa cells develop receptors for follicle-stimulating hormone (FSH), estrogens (E), and androgens (A), rendering the follicle responsive to these hormones. Near the follicular basement membrane, spindle-shaped cells organize into two distinct layers of follicular membrane: the theca interna and theca externa. Theca interna cells possess luteinizing hormone (LH) receptors and gain the ability to synthesize steroid hormones.
Antral Follicle
Under the synergistic effects of estrogen and FSH, the accumulation of follicular fluid among granulosa cells increases, eventually coalescing into a follicular antrum. The diameter of the follicle enlarges to 500 μm, forming an antral follicle. During this stage, late in the previous luteal phase and early in the current follicular phase of the ovarian cycle, serum FSH levels and biological activity increase. When FSH levels exceed a certain threshold, a cohort of antral follicles enters the "growth and development pathway," a process referred to as recruitment. Around the 7th day of the menstrual cycle, among the recruited follicles, the follicle with the lowest FSH threshold preferentially develops into the dominant follicle, while the others gradually degenerate through atresia in a process known as selection. By the 11th–13th day of the menstrual cycle, the dominant follicle enlarges to approximately 18 mm in diameter and secretes increasing amounts of estrogen, raising serum estrogen levels to about 300 pg/ml. Additionally, under FSH stimulation, granulosa cells develop LH and prolactin (PRL) receptors, responding to these hormones. At this stage, the follicle is referred to as a pre-ovulatory follicle.
Preovulatory Follicle
The preovulatory follicle, representing the final stage of follicular development, is a mature follicle also referred to as the Graafian follicle. During this stage, the follicular fluid increases rapidly, leading to an enlarged follicular cavity and a significant increase in follicle size, reaching a diameter of 18–23 mm. The follicle bulges from the ovarian surface and comprises the following structures, listed from outermost to innermost:
- Theca Externa: Composed of dense ovarian stromal tissue, which lacks a clear boundary with the surrounding ovarian stroma.
- Theca Interna: Derived from the stromal cells of the ovarian cortex, consisting of polygonal cells larger than granulosa cells and richly vascularized.
- Granulosa Cells: Cuboidal cells without blood vessels between them, deriving nutrients from the surrounding theca interna.
- Antrum (Follicular Cavity): Filled with a large volume of clear follicular fluid containing estrogen and various bioactive substances that play key regulatory roles in follicular growth and maturation.
- Cumulus Oophorus: Protruding into the follicular cavity in a mound-like structure, and within it, the oocyte is deeply embedded.
- Corona Radiata: A single layer of granulosa cells radiantly arranged around the oocyte.
- Zona Pellucida: A thin, transparent membrane located between the corona radiata and the oocyte.
Ovulation
Ovulation is the process by which the oocyte, along with its surrounding cumulus-oocyte complex (OCCC, also referred to as the oocyte-corona-cumulus complex), is released from the ovary. It involves the completion of the first meiotic division by the oocyte and the breakdown of the follicular wall's collagen layer, resulting in the release of the oocyte.
Before ovulation, the mature follicle secretes estradiol (E2), with circulating levels reaching a peak that induces positive feedback on the hypothalamus (E2 ≥ 200 pg/mL). This triggers a surge in gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to release LH and FSH, resulting in the characteristic LH/FSH surge. The LH surge is a reliable marker of imminent ovulation, occurring approximately 36 hours before follicular rupture.
The LH surge enables the primary oocyte to complete its first meiotic division, forming the first polar body and the mature secondary oocyte. Under the influence of LH, the preovulatory follicle undergoes luteinization and begins producing a small amount of progesterone. Together with LH and FSH, progesterone activates proteolytic enzyme activity within the follicular fluid, leading to the localized digestion of collagen at the apex of the follicular wall, forming a small opening called the stigma.
During the preovulatory phase, prostaglandin levels in the follicular fluid rise significantly, peaking at ovulation. Prostaglandins promote the release of proteolytic enzymes from the follicular wall and trigger smooth muscle contractions within the ovary, facilitating ovulation.
At ovulation, the oocyte is released together with the zona pellucida, the corona radiata, and a small portion of granulosa cells from the cumulus oophorus. Ovulation typically occurs approximately 14 days before the onset of the next menstrual period. It can alternate between ovaries or occur consecutively on the same side. Following release, the oocyte is captured by the infundibulum of the fallopian tube and transported toward the uterus through a combination of peristaltic tubal movements and ciliary action on the tubal epithelium.
Corpus Luteum Formation and Regression
Following ovulation, the release of follicular fluid reduces intracavitary pressure, causing the follicular wall to collapse and fold into numerous wrinkles. Granulosa cells and theca interna cells from the follicular wall invade inward, surrounded by connective tissue from the theca externa, collectively forming the corpus luteum. Under the influence of the LH surge, granulosa cells and theca interna cells undergo luteinization, transforming into granulosa lutein cells and theca lutein cells, respectively.
Both granulosa and theca lutein cells contain carotenoids, and the content of these pigments determines the color intensity of the corpus luteum. The diameter of lutein cells increases from 12–14 μm to 35–50 μm. Under the influence of vascular endothelial growth factor (VEGF), granulosa cells become vascularized, allowing progesterone to enter systemic circulation. By 7–8 days post-ovulation (around the 22nd day of the menstrual cycle), the corpus luteum reaches its peak size and functional activity, measuring 1–2 cm in diameter and appearing yellow. Adequate development and function of the corpus luteum require optimal preovulatory follicular growth, particularly under the stimulation of FSH and sustained LH levels.
If fertilization occurs and the blastocyst implants, the corpus luteum of pregnancy (gravid corpus luteum) enlarges under the influence of human chorionic gonadotropin (hCG) secreted by the trophoblast cells of the embryo. It regresses by the end of the third month of pregnancy when the placenta forms and takes over the production of steroid hormones necessary to sustain the pregnancy.
If fertilization does not occur, or if implantation fails despite fertilization, the non-gravid corpus luteum begins to regress around the 9th–10th day after ovulation. Corpus luteum function ceases after 14 days. While the exact mechanism of regression is not entirely clear, it is thought to involve luteolytic effects mediated by estrogen produced by the corpus luteum itself, along with local ovarian prostaglandins and endothelin-1. During regression, lutein cells shrink and are replaced by connective tissue and fibroblasts, resulting in the formation of the corpus albicans, a white, fibrotic structure.
Following corpus luteum regression, menstruation occurs, and a new follicular cohort begins development in the ovary, initiating a new ovarian cycle.
Synthesis and Secretion of Ovarian Sex Hormones
The primary ovarian sex hormones include estrogens, progestogens, and small amounts of androgens, all of which are steroid hormones. The theca cells and granulosa cells of the follicle are the main sources of estrogen before ovulation, while luteal cells secrete large amounts of progesterone and estrogen after ovulation. Androgens, such as testosterone, are primarily produced by ovarian stromal and hilar cells.
Basic Chemical Structure of Steroid Hormones
Steroid hormones belong to a class of compounds known as steroidal hormones. The basic chemical structure of steroid hormones consists of a cyclopentanoperhydrophenanthrene ring. Steroid hormones can be classified into three groups based on the number of carbon atoms in their structure:
- Those containing 21 carbon atoms are progestogens, with a pregnenane nucleus as their basic structure, such as progesterone.
- Those containing 19 carbon atoms are androgens, with an androstane nucleus as their basic structure, such as testosterone.
- Those containing 18 carbon atoms are estrogens, with an estrane nucleus as their basic structure, such as estradiol, estrone, and estriol.
Biosynthesis of Steroid Hormones
The biosynthesis of ovarian steroid hormones involves multiple hydroxylase and aromatase enzymes, all of which belong to the cytochrome P450 superfamily. Under the stimulation of luteinizing hormone (LH), cholesterol in the theca cells of the follicle is converted into pregnenolone through the enzymatic action of mitochondrial cytochrome P450 side-chain cleavage enzyme. This conversion represents the rate-limiting step in the synthesis of sex hormones. Pregnenolone can then be converted to androstenedione via two pathways: the Δ4 pathway and the Δ5 pathway.
Before ovulation, the ovary synthesizes estrogen primarily via the Δ5 pathway, while both the Δ4 and Δ5 pathways are utilized after ovulation to synthesize estrogen. Progesterone is synthesized exclusively through the Δ4 pathway. Estrogen biosynthesis in the ovary involves the coordinated activity of theca and granulosa cells under the joint influence of follicle-stimulating hormone (FSH) and LH, as outlined in the two-cell, two-gonadotropin theory proposed by Falck in 1959. LH binds to LH receptors on theca cells, leading to the conversion of cholesterol into testosterone and androstenedione, which then serve as precursors of estrogen. These molecules diffuse into granulosa cells, where FSH binds to FSH receptors, activating aromatase. Aromatase converts testosterone and androstenedione into estradiol and estrone, respectively, which are then released into the bloodstream and follicular fluid.
Metabolism of Steroid Hormones
The liver is the primary site for the metabolism of steroid hormones. Metabolites of estradiol include estrone and its sulfates, estriol, and 2-hydroxyestrone, most of which are excreted through the kidneys. A portion of these metabolites is excreted into the intestines via bile, where they may be reabsorbed into the liver through enterohepatic circulation. Progesterone is metabolized primarily into pregnanediol and excreted by the kidneys. Testosterone is metabolized into androsterone and etiocholanolone and is excreted primarily in the form of glucuronides through the kidneys.
Cyclical Changes in Ovarian Hormone Secretion
Estrogen
During the early stages of follicular development, the secretion of estrogen is minimal. By the 7th day of the menstrual cycle, estrogen secretion from the follicle increases rapidly, peaking just before ovulation. Following ovulation, the release of estrogen from the follicular fluid into the peritoneal cavity causes a temporary decline in circulating estrogen levels. Within 1–2 days after ovulation, the corpus luteum begins to secrete estrogen, causing circulating estrogen levels to rise again. Around the 7th–8th day post-ovulation, when the corpus luteum reaches its peak maturity, circulating estrogen levels form a second peak. Thereafter, as the corpus luteum undergoes regression, estrogen levels decline sharply, reaching their lowest levels during menstruation.
Progesterone
Progesterone is not secreted by the follicle during the follicular phase. During the preovulatory LH surge, granulosa cells of the mature follicle undergo luteinization and begin secreting small amounts of progesterone. After ovulation, progesterone secretion by the corpus luteum progressively increases, peaking around the 7th–8th day post-ovulation when the corpus luteum is fully mature. Progesterone levels then gradually decline, returning to follicular-phase levels by the onset of menstruation.
Androgens
Androgens in females are primarily derived from the adrenal glands, with the ovary contributing a portion of androgen production in the form of testosterone, androstenedione, and dehydroepiandrosterone. The theca interna of the follicle is the main site for the synthesis and secretion of androstenedione, while ovarian stromal and hilar cells primarily synthesize and secrete testosterone. Circulating androgen levels increase prior to ovulation. On one hand, androgens promote the atresia of non-dominant follicles; on the other hand, they may enhance sexual desire.
Physiological Functions of Ovarian Sex Hormones
Physiological Effects of Estrogen
Myometrium
Estrogen promotes the proliferation and hypertrophy of uterine muscle cells, leading to thickening of the myometrium. It enhances blood supply and supports the development and maintenance of the uterus. It also increases the sensitivity of uterine smooth muscle to oxytocin.
Endometrium
Estrogen stimulates the proliferation and repair of endometrial glands and stroma.
Cervix
Estrogen promotes relaxation and dilation of the cervix, increases cervical mucus secretion, and makes the mucus thinner, more elastic, and thread-like.
Fallopian Tubes
Estrogen enhances the development of the muscular layer and secretory activity of the epithelial lining. It strengthens the rhythmic contraction amplitude of the fallopian tube muscles.
Vaginal Epithelium
Estrogen induces the proliferation and keratinization of vaginal epithelial cells, thickens the mucosa, and increases intracellular glycogen, which helps maintain the vaginal acidic environment.
External Genitalia
Estrogen stimulates the development and pigmentation of the labia, making them fuller.
Secondary Sexual Characteristics
Estrogen promotes the proliferation of mammary ducts, darkening of the nipple and areola, and supports the development of other secondary sexual characteristics.
Ovaries
Estrogen works in conjunction with FSH to promote follicular development.
Hypothalamus and Pituitary Gland
Estrogen regulates gonadotropin secretion through positive and negative feedback mechanisms on the hypothalamus and pituitary.
Metabolic Functions
Estrogen promotes water and sodium retention, enhances hepatic synthesis of high-density lipoprotein (HDL), inhibits low-density lipoprotein (LDL) synthesis, and reduces blood cholesterol levels. It also supports bone matrix metabolism and maintenance.
Physiological Effects of Progesterone
Progesterone typically exerts its effects based on prior estrogen stimulation.
Myometrium
Progesterone reduces the excitability of uterine smooth muscle and its sensitivity to oxytocin, thereby suppressing uterine contractions. These actions facilitate blastocyst implantation and provide a supportive environment for embryo and fetal growth within the uterus.
Endometrium
Progesterone transforms the proliferative endometrium into secretory endometrium, preparing it for implantation.
Cervix
Progesterone causes the cervix to close, reduces mucus secretion, and makes the mucus thicker.
Fallopian Tubes
Progesterone inhibits the amplitude of rhythmic contractions in the fallopian tubes.
Vaginal Epithelium
Progesterone accelerates the shedding of vaginal epithelial cells.
Breasts
Progesterone promotes the development of mammary alveoli.
Hypothalamus and Pituitary Gland
Progesterone enhances the positive feedback effects of estrogen on the LH peak during ovulation in the mid-menstrual cycle. During the luteal phase, progesterone exerts negative feedback on the hypothalamus and pituitary to inhibit gonadotropin secretion.
Body Temperature
Progesterone stimulates the hypothalamic thermoregulatory center, leading to an increase in basal body temperature by 0.3–0.5°C after ovulation. This temperature change is often used clinically to infer the timing of ovulation.
Metabolic Functions
Progesterone promotes the excretion of water and sodium.
Synergistic and Antagonistic Actions of Progesterone and Estrogen
Progesterone often enhances estrogen-induced effects, further promoting the development of female reproductive organs and breasts in preparation for pregnancy. This denotes their synergistic effects. However, estrogen and progesterone also exhibit antagonistic actions. While estrogen promotes the proliferation and repair of the endometrium, progesterone limits endometrial proliferation and transforms the proliferative endometrium into a secretory phase. Other antagonistic effects include their opposing roles in uterine contraction, fallopian tube motility, cervical mucus changes, vaginal epithelial cell processes (keratinization versus shedding), and water and sodium retention or excretion.
Physiological Effects of Androgens
Effects on the Female Reproductive System
Androgens secretion increases during puberty and supports the development of the clitoris, labia, and mons pubis, as well as the growth of pubic and axillary hair. Excessive androgens can antagonize estrogen, slowing the growth and proliferation of the uterus and endometrium, and inhibiting vaginal epithelial proliferation and keratinization. Androgens also have a role in regulating sexual desire.
Effects on Metabolic Functions
Androgens promote protein synthesis and muscle growth and stimulate red blood cell production in the bone marrow. Before sexual maturity, androgens facilitate the growth of long bone matrix and calcium retention. After sexual maturity, androgens contribute to the closure of the epiphyseal plate, ending longitudinal growth. They also promote reabsorption of water and sodium in the renal distal tubules while aiding in calcium retention.
Mechanism of Action of Steroid Hormones
Steroid hormones are lipid-soluble and primarily enter target cells through diffusion. Within the cell, they bind to cytoplasmic receptors to form hormone-receptor complexes. Steroid hormone receptors in the cytoplasm are proteins that exhibit high affinity and specificity for their corresponding hormones. Upon binding, conformational changes occur in the receptor protein, leading to dissociation of heat shock proteins (HSP). This enables the hormone-receptor complex to translocate into the nucleus, where it binds to nuclear receptors to form hormone-nuclear receptor complexes. These complexes initiate transcription of specific DNA sequences, resulting in the synthesis of mRNA. The mRNA is translated into proteins within the cytoplasmic ribosomes, ultimately producing specific biological effects.
Peptide Hormones Secreted by the Ovary
In addition to steroid hormones, the ovary also secretes various peptide hormones, cytokines, and growth factors.
Peptide Hormones
Three types of peptides can be identified in follicular fluid. Based on their effects on FSH, these peptides are categorized as inhibins, activins, and follistatin (FST). They are produced not only by the ovary but also by the pituitary and other tissues. Together with the ovarian steroid hormone system, they form the activin-inhibin-follistatin system, which regulates the synthesis and secretion of pituitary gonadotropins.
Inhibins
Inhibins are glycoproteins composed of two different subunits (α and β) linked by disulfide bonds. The β subunit can be further classified into βA and βB, forming inhibin A (αβA) and inhibin B (αβB). The primary physiological function of inhibins is to selectively inhibit the production of FSH by the pituitary, affecting its synthesis and secretion. Additionally, inhibins can enhance the activity of LH.
Activins
Activins are dimers composed of two β subunits of inhibins. They exist as activin A (βAβA), activin AB (βAβB), and activin B (βBβB). Recent studies have identified additional β subunits, such as βC, βD, and βE. Activins primarily increase the number of GnRH receptors on pituitary cells via autocrine mechanisms, thereby enhancing the pituitary's responsiveness to GnRH and stimulating the production of FSH.
Follistatin
Follistatin is a highly glycosylated peptide that has an affinity for the β subunits of inhibins and activins. When activins bind to follistatin, their ability to stimulate FSH production is lost. The main function of follistatin is to inhibit FSH production through autocrine and paracrine mechanisms.
Cytokines and Growth Factors
Cytokines and growth factors, such as interleukin-1, tumor necrosis factor-alpha, insulin-like growth factors, vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor, transforming growth factor, and platelet-derived growth factor, also participate in the regulation of follicular growth and development through autocrine and paracrine signaling pathways.