Hematology is an independent branch of medical science that focuses on the study of blood and hematopoietic tissues. The hematological system primarily consists of blood and hematopoietic tissues.
Structure of the Hematological System
Hematopoietic Tissues and Hematopoiesis
Hematopoietic tissues refer to the structures responsible for producing blood cells, including the bone marrow, thymus, lymph nodes, liver, spleen, and hematopoietic tissues during embryonic and fetal development.
Different periods are associated with distinct sites of hematopoiesis, classified into the embryonic, fetal, and postnatal hematopoietic stages: these include the mesoblastic stage, hepatic and splenic stage, and medullary stage. The yolk sac is the earliest site of hematopoiesis during the embryonic stage. After the yolk sac degenerates, the liver and spleen take over hematopoietic functions. Around the fourth to fifth month of fetal development, hematopoiesis in the liver and spleen begins to decline as hematopoiesis begins in the bone marrow, thymus, and lymph nodes. Post-birth, these organs continue their hematopoietic functions. After puberty, the thymus gradually atrophies, while lymph nodes produce lymphocytes and plasma cells. Bone marrow becomes the primary site of hematopoiesis after birth. However, when bone marrow is unable to meet hematopoietic demands, other organs such as the liver and spleen may participate in what is referred to as extramedullary hematopoiesis.
Hematopoietic Cell Generation and Regulation of Hematopoiesis
It is widely accepted that all hematopoietic cells and immune cells originate from common hematopoietic stem cells (HSCs) in the bone marrow. These HSCs are characterized by their ability for self-renewal and multi-lineage differentiation.
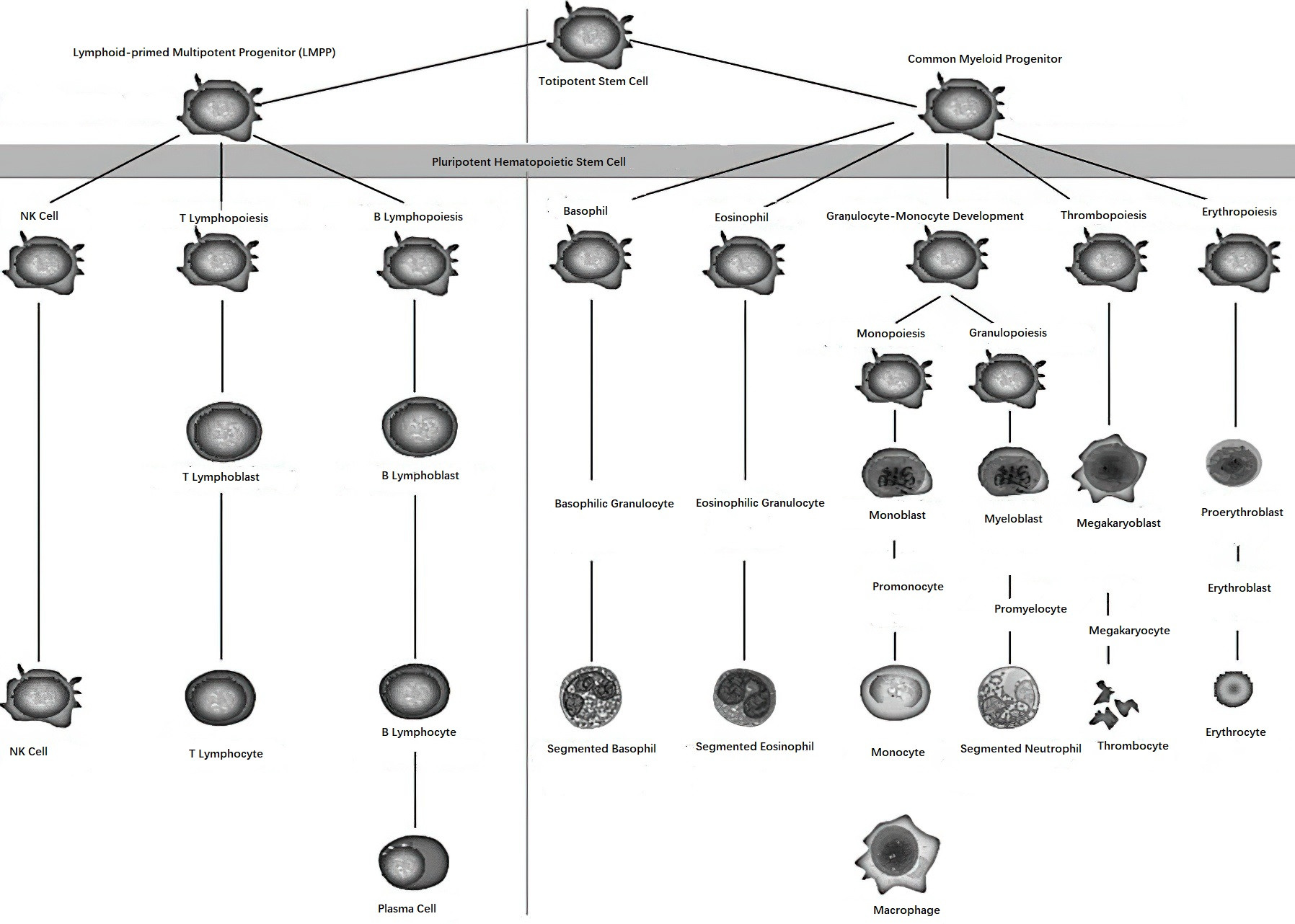
Figure 1 Schematic diagram of hematopoiesis
HSCs can be identified based on specific surface antigens. Multipotent HSCs are mainly represented by the CD34+ cell population, which lacks lineage-specific antigens (known as Lin antigens). As hematopoietic stem cells differentiate and mature, the expression of the CD34 antigen gradually decreases. Myeloid progenitor cells express antigens such as CD34 and CD33, while lymphoid progenitor cells express CD34 in addition to CD38 and HLA-DR antigens.
Studies have shown that CD34+ cells account for 1% of nucleated cells in the bone marrow and approximately 0.05% in peripheral blood.
Hematopoiesis requires not only HSCs but also a normal hematopoietic microenvironment and the presence of regulatory factors, both positive and negative. Non-hematopoietic components within hematopoietic tissues, including the microvascular system, neural elements, reticular cells, stroma, and other connective tissue, collectively constitute the hematopoietic microenvironment. This microenvironment can directly interact with hematopoietic cells or release certain factors to influence or induce the generation of hematopoietic cells. Regulatory factors involved in hematopoiesis include humoral factors that stimulate the proliferation of various progenitor cells (positive regulators), such as erythropoietin (EPO), colony-stimulating factors (CSFs), and interleukin-3 (IL-3). There are also negative regulators, such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). These positive and negative factors work together to maintain the homeostasis of hematopoietic functions.
Classification
Hematological diseases refer to conditions that are primarily associated with or directly involve blood and hematopoietic organs, such as primary conditions like leukemia or secondary involvement as in iron-deficiency anemia. The classification of hematological diseases is as follows:
Diseases of Red Blood Cells
These include various types of anemia and polycythemia.
Diseases of Granulocytes
Examples include agranulocytosis, neutrophil segmentation dysfunction (Pelger-Huët anomaly), lazy leukocyte syndrome, and leukemoid reactions.
Diseases of Monocytes and Macrophages
These include conditions such as inflammatory histiocytosis.
Diseases of Lymphocytes and Plasma Cells
Conditions in this category include various types of lymphoma, acute and chronic lymphocytic leukemia, hemophagocytic lymphohistiocytosis (HLH), and multiple myeloma.
Diseases of Hematopoietic Stem Cells
These include disorders such as aplastic anemia, paroxysmal nocturnal hemoglobinuria, myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), and acute myeloid leukemia (AML).
Hypersplenism
Hemorrhagic and Thrombotic Disorders
This category includes vascular purpura, thrombocytopenic purpura, coagulation disorders, disseminated intravascular coagulation (DIC), and thrombotic diseases.
Hematology encompasses not only hematological diseases but also transfusion medicine and hematopoietic stem cell transplantation.
Diagnosis
Hematological diseases exhibit many characteristics that distinguish them from other diseases, which are determined by the unique features of blood and hematopoietic tissues. Since blood exists in liquid form and continuously circulates throughout the body, perfusing the microcirculation of every organ, hematological diseases often present with systemic manifestations. Additionally, blood is a composite system consisting of blood cells and plasma components that perform various physiological functions and work in concert with hematopoietic tissues to maintain a dynamic balance. The symptoms and signs of hematological diseases are therefore diverse and often lack specificity. Laboratory tests play a prominent role in the diagnosis of hematological diseases. Secondary hematological abnormalities are more common than primary hematological diseases, as pathological changes in nearly all organs and tissues may result in alterations in blood parameters. In some cases, such abnormalities may mimic those of primary hematological diseases, exhibiting severe and persistent changes.
Medical History Collection
Common symptoms of hematological diseases include anemia, bleeding tendencies, fever, masses, hepatosplenomegaly, lymphadenopathy, and bone pain. Patient history should include the presence or absence of these symptoms along with their characteristics. Additional information such as a history of exposure to drugs, toxins, or radioactive substances, dietary habits, surgical history, menstrual history, pregnancy and childbirth history, and family history should also be obtained.
Physical Examination
Observations include changes in skin and mucosal color, the presence of jaundice, petechiae, nodules, or plaques, and abnormalities in tongue papillae. Other aspects of the examination involve assessing for sternal tenderness, enlargement of superficial lymph nodes, liver or spleen, abdominal masses, and other physical findings.
Laboratory Examinations
Blood Cell Counts and Morphology
Accurate blood cell counts, hemoglobin measurements, and detailed examination of blood smears under a microscope are the most fundamental diagnostic methods. These often reflect pathological changes in bone marrow hematopoiesis.
Reticulocyte Counts
This test reflects the functionality of red blood cell production in the bone marrow.
Bone Marrow Examination and Cytochemical Staining
Bone marrow aspiration smears, trephine biopsies, and cytochemical staining techniques contribute to confirming certain hematological diseases such as leukemia, multiple myeloma, and myelofibrosis. They also provide supportive information for other conditions, such as hyperplastic anemia. Cytochemical stains, including myeloperoxidase, alkaline phosphatase, and non-specific esterase stains, are essential for the differential diagnosis of acute leukemia.
Hemorrhagic Disease Tests
Basic tests include bleeding time, clotting time, prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen level measurement. Platelet function tests, such as clot retraction, platelet aggregation, and adhesion tests, along with coagulation factor assays, help evaluate platelet functionality and coagulation factor activity.
Tests for Hemolytic Diseases
Commonly used tests include free hemoglobin measurement, haptoglobin analysis, and the Rous test. Intravascular hemolysis may be assessed with tests for occult blood in urine. Paroxysmal nocturnal hemoglobinuria may be diagnosed through acidified serum lysis and sucrose hemolysis tests. Hereditary spherocytosis can be evaluated with osmotic fragility tests. Glucose-6-phosphate dehydrogenase deficiency can be confirmed with methemoglobin reduction tests. Autoimmune hemolytic anemia can be diagnosed using the Coombs test (direct antiglobulin test).
Biochemical and Immunological Tests
Iron metabolism tests are performed for iron-deficiency anemia. In autoimmune and lymphatic disorders, abnormalities in immunoglobulin levels, cellular immunity, and anti-blood cell antibodies are commonly observed. Immunophenotyping with specific monoclonal antibodies is now one of the diagnostic standards for acute leukemia. Immunohistochemistry is a necessary component in diagnosing lymphoma.
Cytogenetic and Molecular Biological Tests
Chromosomal analysis and genetic testing play important roles in the diagnosis of hematological diseases.
Hematopoietic Cell Culture and Detection Techniques
Imaging Studies
Imaging techniques such as ultrasound, CT, and MRI are routinely used. Advanced technologies like SPECT and PET-CT are valuable for visualizing bone marrow, lymph nodes, liver, spleen, and other extramedullary lesions, significantly aiding the diagnosis of hematological diseases.
Radioisotope Analysis
This technique is applied in determining red blood cell lifespan.
Histopathological Examination
Examinations include biopsies of lymph nodes or infiltrated masses, spleen biopsies, and cytology of bodily fluids. Lymph node biopsies are particularly important in the diagnosis of lymphoma and the distinction between lymphomas, lymphadenitis, and metastatic carcinomas. Spleen biopsies are primarily used in conditions involving marked splenomegaly. Cytological examinations of bodily fluids, such as pleural effusions, ascitic fluid, and cerebrospinal fluid, are valuable for diagnosing the presence of tumor cells or leukemic cells, as well as for treatment planning and prognosis evaluation.
Laboratory tests for hematological diseases are extensive and require comprehensive analysis. A holistic approach should be taken to select appropriate tests to achieve a definitive diagnosis.
Treatment
General Treatment
The approach includes diet and nutrition management as well as psychological and mental health care.
Removal of Causative Factors
Efforts focus on eliminating the patient's exposure to the factors causing the disease.
Maintenance of Normal Blood Composition and Function
Supplementation of Nutrients Needed for Hematopoiesis
For megaloblastic anemia, supplementation with folic acid and/or vitamin B12 is required. Iron supplementation is administered in cases of iron-deficiency anemia.
Stimulation of Hematopoiesis
For instance, androgens may be used to stimulate hematopoiesis in chronic aplastic anemia, while granulocyte colony-stimulating factor (G-CSF) can be employed to stimulate the release of neutrophils during granulocytopenia.
Splenectomy
Splenectomy involves the removal of the largest organ in the mononuclear phagocyte system to reduce blood cell destruction and sequestration, thereby prolonging blood cell lifespan. This procedure is particularly effective in treating hemolytic anemia caused by hereditary spherocytosis.
Adoptive Immunotherapy
Therapies may include the administration of interferons or donor lymphocyte infusions (DLI) following allogeneic hematopoietic stem cell transplantation.
Component Transfusion and Antibiotics Usage
Red blood cell transfusions are provided in cases of severe anemia or blood loss. Platelet transfusions are given in cases of thrombocytopenia with a risk of bleeding. Effective anti-infective medications are used in patients with leukopenia and infections.
Removal of Abnormal Blood Components and Inhibition of Abnormal Function
Chemotherapy
Combination regimens employing chemotherapeutic drugs targeting different stages of the cell cycle are utilized to kill diseased cells.
Radiotherapy
Ionizing radiation, such as gamma rays or X-rays, is used to destroy leukemia or lymphoma cells.
Induced Differentiation
Scientists have discovered that agents such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) can induce the differentiation of abnormal promyelocytes, accelerating their apoptosis or maturation into normal granulocytes. This represents a novel and targeted approach to eliminating leukemic cells.
Therapeutic Apheresis
Using a blood cell separator to selectively remove specific components from the blood is effective in treating conditions such as myeloproliferative neoplasms (MPNs) and leukemia. Plasma exchange is helpful for treating diseases like Waldenström’s macroglobulinemia, certain autoimmune diseases, alloimmune diseases, and thrombotic thrombocytopenic purpura (TTP).
Immunosuppression
Treatment involves the use of glucocorticoids, cyclosporine, and anti-lymphocyte or anti-thymocyte globulins to reduce lymphocyte counts and inhibit their abnormal functions. These therapies are used for conditions such as autoimmune hemolytic anemia, aplastic anemia, and graft-versus-host disease (GVHD) in the context of allogeneic hematopoietic stem cell transplantation.
Anticoagulation and Thrombolytic Therapy
In cases of disseminated intravascular coagulation, heparin is used to prevent further depletion of clotting factors. Medications like dipyridamole are used to prevent abnormal platelet aggregation in thrombocytosis. Thrombolytics such as urokinase are employed when thrombosis occurs to restore proper blood flow.
Targeted Therapy
Targeted drugs, such as tyrosine kinase inhibitors, are used in the treatment of chronic myeloid leukemia (CML).
Epigenetic Inhibition
Oral histone deacetylase (HDAC) inhibitors like chidamide are used for the treatment of relapsed and refractory peripheral T-cell lymphoma. Hypomethylating agents like decitabine are used as first-line treatments for older patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).
Hematopoietic Stem Cell Transplantation (HSCT)
This involves preconditioning to remove abnormal bone marrow hematopoietic tissues, followed by transplantation of healthy hematopoietic stem cells. This allows for the reconstruction of the hematopoietic and immune systems, making HSCT a comprehensive treatment option with the potential to cure hematological malignancies and genetic disorders.
Cellular Immunotherapy
Chimeric antigen receptor T-cell (CAR-T) immunotherapy has shown significant efficacy in the treatment of acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma.
Advances and Outlook in Hematology
Over the last decade, hematology, especially the study of hematological malignancies, has emerged as one of the most prominent fields in contemporary medical research. Since the discovery of blood cells in the 18th century, nearly 300 years of integration between basic and clinical research has propelled the field into a new era. Since leukemia was first identified in the 19th century, the cure rate for childhood ALL and adult acute promyelocytic leukemia (APL) has reached approximately 75% in the 21st century.
The diagnostic approach to hematological malignancies has evolved from a morphology-based paradigm to advanced stages involving molecular biology and genomics. Therapies have progressed from conventional chemotherapy to include induced differentiation, targeted gene therapy, HSCT, and cellular immunotherapy, making hematology a paradigm-shifting model for treating malignancies.
Looking ahead, the development of new therapeutic targets, alongside advancements in areas such as biological-effect-based treatments and gene therapy, will continue to shape the future of the field. The advancement of hematology is poised to drive progress in other areas of medicine as well.