The urinary system consists of the kidneys, ureters, bladder, urethra, prostate (in males), and associated blood vessels and nerves. Its primary functions include filtration (producing and excreting urine to eliminate metabolic waste and water from the body); reabsorption and secretion (regulating internal homeostasis, maintaining electrolyte and acid-base balance); and endocrine functions (regulating blood pressure, erythropoiesis, as well as calcium, phosphorus, and bone metabolism).
Anatomical Structure of the Kidneys
Humans have two kidneys, one on each side, located retroperitoneally on either side of the spine, approximately between the 12th thoracic vertebra (T12) and the 3rd lumbar vertebra (L3). The right kidney is positioned 0.5 to 1 vertebral body lower than the left kidney. In adults, the size of the kidneys measures approximately 10.5–11.5 cm in length, 5.0–7.2 cm in width, and 2.0–3.0 cm in thickness, resembling the shape of a kidney bean. The weight of a single kidney is 100–140 g in males, slightly lighter in females. The outer margin of the kidney is convex, while the inner margin is concave, with the central area of the concavity known as the renal hilum. The renal hilum serves as the entry and exit point for blood vessels, lymphatic vessels, ureters, and nerves. On a coronal section of the kidney, the renal parenchyma is divided into the outer renal cortex and the inner renal medulla. Renal medulla forms renal pyramids, with their bases facing the renal cortex and their apices extending toward renal papillae.
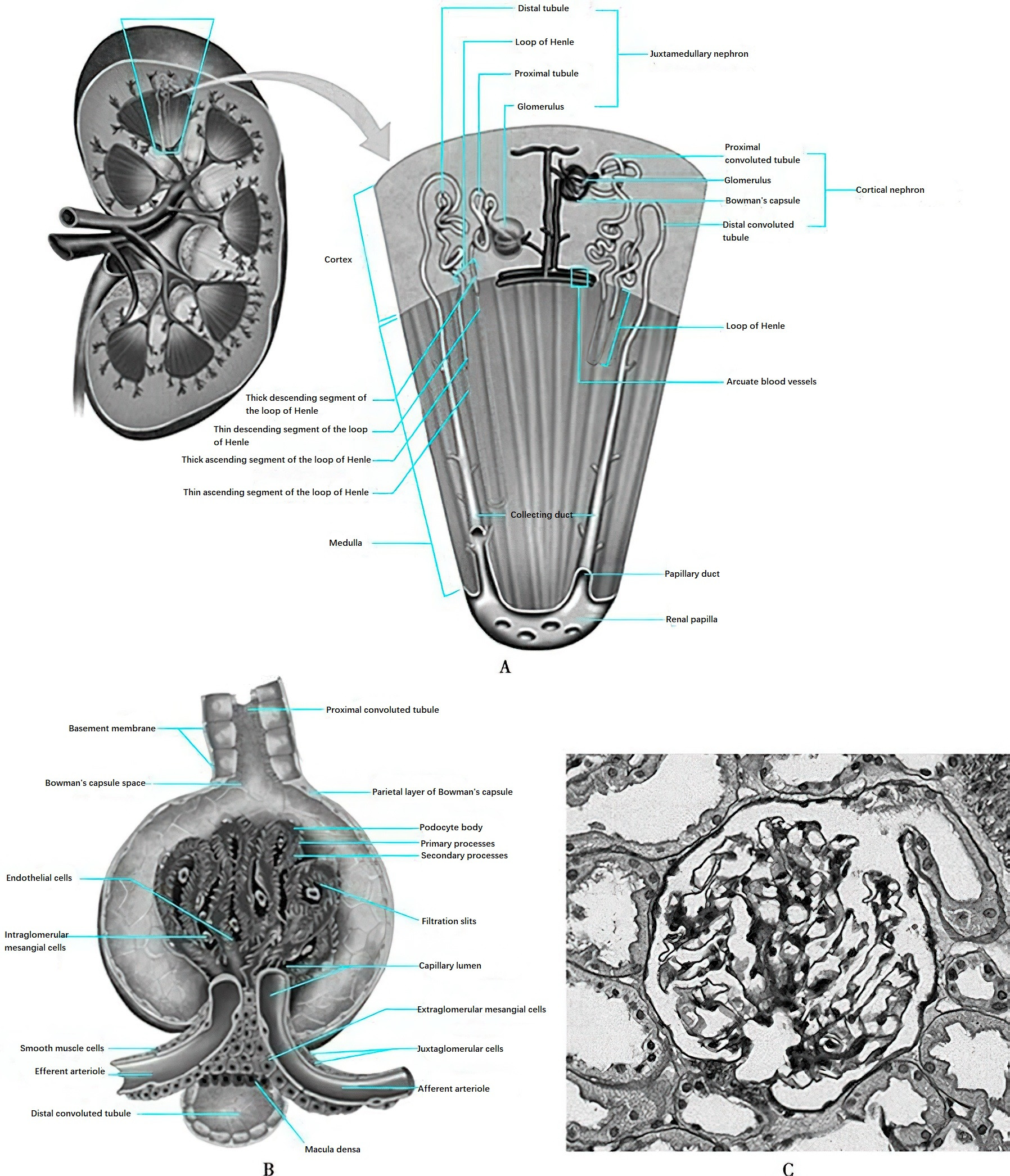
Figure 1 Structure of the nephron
The nephron is the fundamental structural and functional unit of the kidney, with each kidney containing approximately 1 million nephrons. A nephron consists of two main components: the renal corpuscle and the renal tubule. The renal corpuscle is composed of the glomerular capillary network and the surrounding glomerular capsule (Bowman’s capsule). The afferent arteriole delivers blood to the glomerulus, while the efferent arteriole carries blood away. The glomerulus is a critical component of the nephron, with the term "glomerulus" often used to refer to the entire renal corpuscle. The glomerular capillary network comprises three main types of cells (endothelial cells, visceral epithelial cells, and mesangial cells), the basement membrane, and the mesangial matrix.
Endothelial cells are flattened cells lining the inner walls of the capillaries, containing numerous fenestrations (pores), which serve as the first layer of the glomerular filtration barrier. These cells carry a negative charge and, together with the glomerular basement membrane (GBM) and the foot processes of visceral epithelial cells, form the filtration barrier of the glomerulus. The GBM has a thickness of 270–350 nm and functions as a selective semipermeable membrane. Under an electron microscope, it consists of three layers: the inner loose layer, the dense layer, and the outer loose layer. Visceral epithelial cells, also known as podocytes, have extensive foot-like projections called foot processes. These foot processes interdigitate, forming filtration slits between them, which attach to the basement membrane. Podocytes are terminally differentiated cells that play a critical role in maintaining the integrity of the glomerular filtration barrier. Podocyte-associated proteins, such as nephrin, podocin, and podocalyxin, constitute the molecular sieve of the filtration barrier and are crucial to the filtration function. Abnormalities in these proteins can compromise the structure and stability of the filtration barrier, resulting in proteinuria.
The mesangium, located between the glomerular capillaries, consists of mesangial cells and mesangial matrix. It provides structural support for the glomerular capillary network and plays a role in regulating the glomerular filtration rate.
The renal tubule consists of the proximal tubule, descending and ascending limbs of the loop of Henle, distal tubule, and collecting duct. The collecting duct gathers the formed urine and transports it through the renal papilla into the renal calyx, eventually into the ureter. Different segments of the renal tubule are lined by highly specialized epithelial cells, which vary significantly in morphology and function and exhibit distinct polarity. The distribution of various transport proteins on the luminal side and the basolateral side of the tubule forms the structural and functional basis for the directional transport of water and solutes.
The juxtaglomerular apparatus (JGA) is located at the vascular pole of the glomerulus and consists of the macula densa, juxtaglomerular cells, extraglomerular mesangial cells, and polar cap cells. Juxtaglomerular cells are derived from the smooth muscle cells of the afferent and efferent arterioles at the vascular pole and exhibit epithelial-like characteristics. The macula densa is made up of tall columnar cells, differentiated from the epithelial cells of the distal tubule that are adjacent to the glomerulus near the vascular pole. The macula densa is situated at the angle formed by the afferent and efferent arterioles and senses the sodium concentration in the tubular fluid. Through regulating renin release from juxtaglomerular granular cells, it adjusts the vascular tone of the afferent arteriole, thereby modulating the glomerular filtration rate. This mechanism is referred to as the tubuloglomerular feedback.
Physiological Functions of the Kidneys
The kidneys perform several vital physiological functions, including excretion of metabolic waste products, regulation of water, electrolytes, and acid-base balance to maintain internal homeostasis, as well as endocrine activities.
Glomerular Filtration Function
The kidneys receive approximately 25% of the cardiac output. Filtration is the most important physiological function of the kidneys, and the glomerular filtration rate (GFR) is the parameter most commonly used to assess kidney function clinically. In resting adults, the GFR is about 120 ml/(min·1.73 m2) in men and approximately 10% lower in women. The GFR correlates with age, peaking between 25 and 30 years and gradually declining thereafter. GFR is primarily influenced by factors such as renal blood flow, effective filtration pressure, glomerular membrane surface area, and capillary permeability.
Tubular Reabsorption and Secretion Functions
The glomeruli filter approximately 180 liters of primary urine per day, of which 99% of water, all glucose and amino acids, most electrolytes, and bicarbonate are reabsorbed by the renal tubules and collecting ducts, forming around 1.5 liters of final urine daily. The proximal tubule is the main site of reabsorption, where all filtered glucose and amino acids are reabsorbed. Sodium (Na+) is actively reabsorbed through the Na+-K+-ATPase pump, along with bicarbonate (HCO3-) and chloride (Cl-). In addition to reabsorption, the proximal tubule also plays a role in the secretion of organic acids. Uric acid is filtered by the glomeruli but is mostly reabsorbed in the renal tubules before being secreted back into the tubular lumen. This process also applies to drugs such as antibiotics and contrast agents.
The loop of Henle plays a crucial role in maintaining the osmotic gradient of the medulla. Water can freely pass through the thin descending limb of the loop of Henle, while Na+ and Cl- cannot, leading to rapid water reabsorption in the hyperosmotic medullary region. In the ascending limb, however, Na+ and Cl- can pass through while water cannot, sustaining the high osmolality of the medullary region. This makes the loop of Henle critical for the kidney's urine concentration function. The distal tubule, particularly the connecting tubule, is a key site for regulating the final composition of urine. The epithelial cells here reabsorb Na+, secrete K+, and excrete H+ and NH4+. Aldosterone enhances these activities.
Endocrine Functions of the Kidneys
The kidneys perform important endocrine functions, including the synthesis and secretion of renin, erythropoietin (EPO), 1,25-dihydroxyvitamin D₃, prostaglandins, and kinins. These substances regulate hemodynamics, erythropoiesis, calcium and phosphorus metabolism, and bone metabolism.
The production of EPO by the kidneys is regulated by the oxygen content of local tissues in the renal cortex and outer medulla. EPO is secreted into the bloodstream and acts on erythroid progenitor cells in the bone marrow to stimulate red blood cell production.
The kidneys are also the primary site of 1α-hydroxylase production, an enzyme that converts 25-hydroxyvitamin D3 into 1,25-dihydroxyvitamin D3, the most biologically active form of vitamin D. 1,25-dihydroxyvitamin D3 maintains calcium and phosphorus homeostasis and proper bone mineralization by regulating calcium and phosphorus absorption in the gastrointestinal tract, renal excretion, bone remodeling, and parathyroid hormone secretion.
Clinical Manifestations of Kidney Diseases
The clinical manifestations of kidney diseases include symptoms directly related to the kidney as well as systemic symptoms caused by kidney dysfunction. These may include abnormal urine color, changes in urine volume, edema, fatigue, and other symptoms. Secondary kidney diseases may also present with signs of the primary disease and damage to other organs, such as rashes, joint pain, oral ulcers, or hair loss.
Hematuria
Hematuria can be classified as gross hematuria (visible to the naked eye) and microscopic hematuria. Microscopic hematuria is defined as more than 3 red blood cells per high-power field in a fresh urine sediment examination without visible changes in urine color. Gross hematuria is characterized by darkened, red-colored urine or urine resembling "washing meat water."
Proteinuria
Proteinuria often presents as increased foam in the urine and is defined as a positive urinary protein dipstick test or a urinary protein excretion exceeding 150 mg/day.
Edema
Edema is a common clinical manifestation of kidney diseases. Renal edema often occurs in loose tissues, such as the eyelids, or in dependent areas such as the ankles and shins. In bedridden patients, edema is most evident in the sacral and coccygeal regions.
Hypertension
Hypertension is a common feature of kidney diseases, and all patients with hypertension should be screened for underlying kidney disease, particularly younger individuals. Renal hypertension is categorized into renovascular hypertension and renal parenchymal hypertension. Sodium and water retention is the primary mechanism in renal parenchymal hypertension, with the renin-angiotensin-aldosterone system also playing a significant role in its development.
Examinations for Kidney Diseases
Examinations for kidney diseases primarily include urinalysis, kidney function tests, imaging studies, and pathological examinations of the kidney.
Urinalysis
Routine Urinalysis
Routine urinalysis includes assessments of urine appearance, physical and chemical tests, urine sediment examination, and biochemical tests. This is an important tool for early detection and diagnosis of kidney diseases. Fresh, clean urine samples are required to avoid contamination or degradation due to prolonged storage.
Phase-Contrast Microscopy of Urine
Phase-contrast microscopy is used to determine the origin of red blood cells in urine. Glomerular hematuria is characterized by dysmorphic red blood cells, with more than 5% spiky-shaped red blood cells or a predominance of deformed red blood cells in the sample. The presence of red blood cell casts in urine is indicative of glomerular hematuria.
Urine Protein Testing
Quantitative Proteinuria
This can be assessed using two primary methods:
A 24-hour urine protein level exceeding 150 mg qualifies as proteinuria, and levels greater than 3.5 g indicate nephrotic-range proteinuria.
The albumin-to-creatinine ratio (ACR) in a random urine sample is also used, with normal levels being <30 mg/g, values between 30–300 mg/g indicating microalbuminuria, and values >300 mg/g considered proteinuria. If ACR is significantly elevated (500–1000 mg/g), total protein-to-creatinine ratio (PCR) may also be measured. While 24-hour urine collection offers greater accuracy, it is time-consuming and prone to incomplete collection. Random urine sample testing is easier but subject to variables such as posture and physical activity. These factors should be taken into account when choosing a method or interpreting results.
Albuminuria Testing
In conditions like diabetes that cause kidney damage, increased urinary albumin excretion often precedes an increase in total urinary protein. Testing includes 24-hour urinary albumin quantification or ACR in random samples.
Other Urinary Components Testing
Components such as urinary β2-microglobulin assess proximal tubular reabsorption, while κ or λ light chains aid in diagnosing paraproteinemias. Urinary sodium testing reflects dietary salt intake, urinary potassium testing assists in diagnosing renal tubular acidosis and hypokalemia, and urinary urea nitrogen levels can estimate dietary protein intake and assess nutritional status.
Kidney Function Tests
Serum Creatinine (Scr) Testing
Serum creatinine testing is the most commonly used method to clinically assess glomerular filtration. Despite its convenience and rapid results, it has low sensitivity and does not indicate early kidney damage; serum creatinine levels typically increase only after 50% of glomerular filtration capacity is lost. Factors such as sex, age, muscle mass, dietary protein intake, and certain medications (e.g., cimetidine) influence serum creatinine levels.
Estimated Glomerular Filtration Rate (eGFR)
Formulas used to estimate GFR include the Cockcroft-Gault formula, the MDRD formula, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Cockcroft-Gault Formula
This formula is not standardized to isotope-dilution mass spectrometry (IDMS) for serum creatinine measurement and is no longer recommended for clinical use.
CCr = {[(140 - Age) × Body Weight] / (72 × Scr)} × 0.85 (for women)
CCr represents creatinine clearance, Age in years, Body Weight in kg, Scr in mg/dL.
MDRD Formula
The MDRD formula is more accurate than CKD-EPI for eGFR values <60 ml/(min·1.73 m2).
eGFR = 175 × Scr – 1.154 x Age - 0.203 × 0.742 (for women) × 1.210 (for African Americans)
CKD-EPI Formula
This is the currently recommended formula for assessing GFR. The updated CKD-EPI 2021 formula excludes race as a variable.
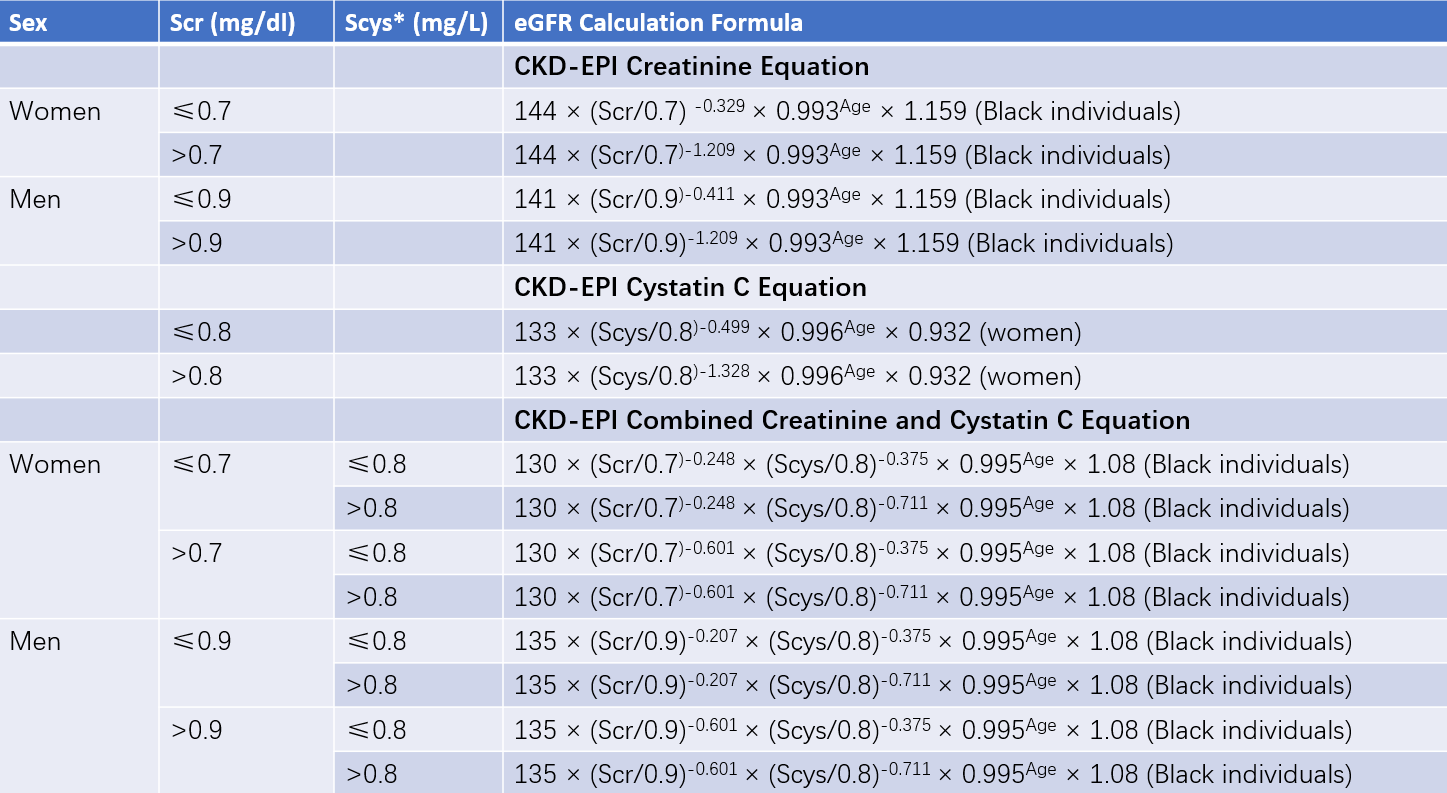
Table 1 CKD-EPI equation
Note: * represents serum Cystatin C (mg/L).
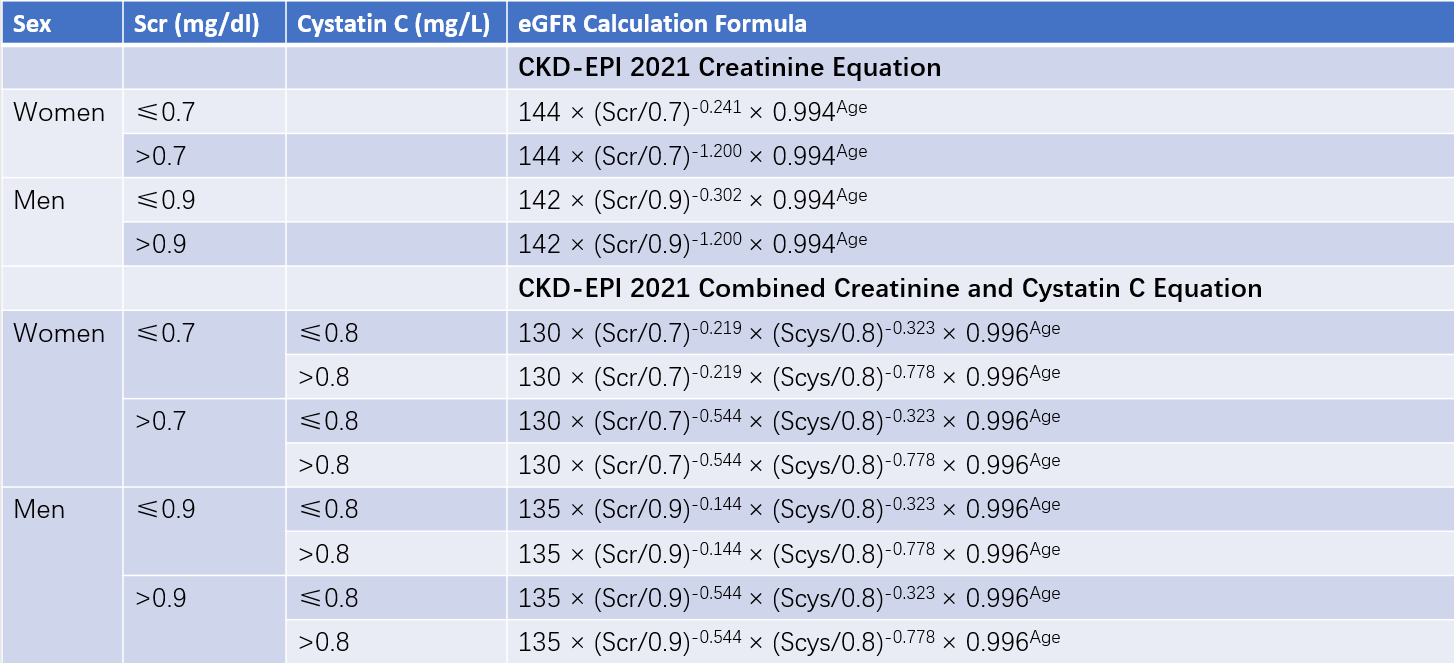
Table 2 CKD-EPI 2021 equation
Note: The CKD-EPI 2021 equation does not include race as a variable.
Endogenous Creatinine Clearance Rate
This is calculated using serum creatinine concentration and 24-hour urinary creatinine excretion. Because urinary creatinine is partly secreted by renal tubules, endogenous creatinine clearance tends to overestimate GFR. However, this method is still used to evaluate residual kidney function in patients undergoing hemodialysis or peritoneal dialysis.
Inulin Clearance and Isotope Measurements
Inulin clearance was historically regarded as the "gold standard" for GFR determination. However, the labor-intensive nature of the method limits routine clinical application, and it is mainly used in research. Isotope-based methods for measuring GFR, such as using 99mTc-labeled compounds, are now more commonly employed in clinical practice due to their accuracy, which is comparable to inulin clearance.
In terms of accuracy, the methods for assessing GFR rank from highest to lowest as follows: inulin clearance, isotope-based measurement, endogenous creatinine clearance, eGFR, and serum creatinine. High-accuracy methods are preferred for high-risk populations or patients with kidney disease to minimize the risk of missed diagnoses.
Imaging Studies
Imaging studies include ultrasonography, intravenous urography, computed tomography (CT), magnetic resonance imaging (MRI), renal angiography, and radionuclide scans.
Renal Pathological Examination
Tissue samples for pathological examination of kidney diseases are often obtained through percutaneous renal biopsy. Although invasive, this procedure is invaluable for diagnosing kidney diseases, assessing disease severity, predicting prognosis, and guiding treatment. Renal biopsy specimens are typically analyzed using three techniques: light microscopy, immunofluorescence, and electron microscopy. Special stains (e.g., Congo red) may be used for specific examinations. The pathological diagnosis integrates analyses of glomerular, tubular, interstitial, and vascular changes with clinical manifestations to provide a definitive diagnosis.
Common Syndromes of Kidney Diseases
Kidney diseases often present as clinical syndromes, which may overlap with one another.
Nephrotic Syndrome (NS)
This is characterized by significant proteinuria (>3.5 g/day), hypoalbuminemia (<30 g/L), and is often accompanied by edema and/or hyperlipidemia. Nephrotic syndrome may arise from primary glomerular diseases (such as minimal change disease, membranous nephropathy, and focal segmental glomerulosclerosis) or secondary glomerular diseases (such as diabetic nephropathy and lupus nephritis).
Nephritic Syndrome
This syndrome is predominantly marked by glomerular hematuria, often accompanied by proteinuria. It can include manifestations such as edema, hypertension, and/or kidney function impairment. Depending on the onset and progression, nephritic syndrome can be classified into three types:
- Acute Nephritic Syndrome: This type has an acute onset, often seen in children, and is frequently preceded by infections such as acute tonsillitis or skin infections. Post-streptococcal acute glomerulonephritis is the most typical example.
- Rapidly Progressive Nephritic Syndrome: The main feature is rapidly progressive kidney function impairment within a short period. It can occur in conditions such as anti-glomerular basement membrane disease, ANCA-associated vasculitis, severe lupus nephritis, and IgA nephropathy.
- Chronic Nephritic Syndrome: This type has an insidious onset, with early symptoms such as edema and fatigue often being mild or absent. Persistent or progressively worsening hematuria and proteinuria are observed. Hypertension and/or kidney function impairment may develop as the disease progresses.
Asymptomatic Hematuria and/or Proteinuria
This refers to hematuria and/or mild to moderate proteinuria without accompanying symptoms such as edema or hypertension. It is frequently seen in various primary glomerular diseases (e.g., IgA nephropathy) and tubulointerstitial disorders.
Acute Kidney Injury (AKI)
AKI refers to a rapid decline in kidney function, characterized by an increase in serum creatinine by ≥26.5 μmol/L within 48 hours, an increase of ≥50% relative to baseline, or a urine output of <0.5 ml/(kg·h) for more than 6 hours. Clinically, it primarily manifests as oliguria or anuria, retention of nitrogenous waste products, and disturbances in water, electrolyte, and acid-base balance. The most critical aspect in AKI diagnosis is distinguishing between prerenal, intrinsic, and postrenal causes.
Chronic Kidney Disease (CKD)
CKD is defined as kidney damage or a glomerular filtration rate (GFR) <60 ml/(min·1.73 m²) lasting for more than 3 months. Kidney damage includes abnormalities seen on renal pathology, abnormal markers of kidney injury in blood or urine, electrolyte imbalances caused by tubular dysfunction, abnormalities on kidney imaging studies, or a history of kidney transplantation. End-stage renal disease (ESRD) represents the most severe stage of CKD, clinically presenting with gastrointestinal symptoms, cardiovascular complications, anemia, and renal osteodystrophy.
Diagnosis of Kidney Diseases
The diagnosis of kidney diseases should include etiological, pathological, functional, and complication assessments to accurately reflect the nature and severity of the disease. This provides a foundation for selecting treatment strategies and determining prognosis.
Etiological Diagnosis
The first step is to differentiate between primary and secondary kidney diseases. Primary kidney diseases include immune-mediated nephritis, infectious diseases of the urinary tract, renal vascular diseases, kidney stones, renal tumors, and congenital kidney disorders. Secondary kidney diseases may result from conditions such as tumors, metabolic disorders, autoimmune diseases, or nephrotoxic damage caused by drugs or toxins.
Pathological Diagnosis
For conditions such as nephritis, nephrotic syndrome, acute kidney injury, and unexplained proteinuria and/or hematuria, renal biopsy can help identify the pathological type and etiology, guide treatment, and assess prognosis.
Functional Diagnosis
For patients diagnosed with AKI or CKD, staging of kidney function is necessary. AKI is classified into stages 1–3 based on changes in serum creatinine and urine output, as detailed in Chapter 9 of this text. CKD is categorized into stages 1–5 according to the degree of GFR decline.
Complication Diagnosis
Kidney diseases, particularly acute and chronic kidney failure, can lead to systemic complications affecting the central nervous system, respiratory system, circulatory system, and others.
Principles of Prevention and Management of Kidney Diseases
Management of kidney diseases depends on the etiology, pathogenesis, affected site, pathological diagnosis, and functional assessment. Therapeutic strategies include the removal of precipitating factors, general management, etiological and pathogenetic treatment, management of coexisting conditions and complications, and renal replacement therapy.
General Treatment
General treatment includes avoiding excessive physical exertion, eliminating triggers such as infections, and reducing exposure to nephrotoxic drugs or toxins. It also involves adopting a healthy lifestyle, such as cessation of smoking, limiting alcohol consumption, balancing rest and exercise, and managing emotional well-being, along with following a sensible diet.
Etiological and Pathogenesis-Based Treatments
Immunosuppressive Therapy
For kidney diseases, particularly immune-mediated primary and secondary glomerular diseases such as IgA nephropathy and lupus nephritis, abnormal immune responses are a key pathogenic mechanism. Treatment often involves glucocorticoids and immunosuppressants. Certain blood purification therapies, such as immunoadsorption and plasma exchange, are effective in removing autoantibodies and antigen-antibody complexes from the body. These therapies are particularly beneficial for severe immune-related kidney diseases, including severe lupus nephritis and vasculitis-associated kidney damage.
Treatment Targeting Non-Immune Mechanisms
Non-immune factors such as hypertension, hyperlipidemia, hyperglycemia, hyperuricemia, and proteinuria play significant roles in the onset and progression of kidney diseases. Interventions addressing these factors are important measures for preserving kidney function.
Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) can inhibit the overactivation of the renin-angiotensin system within the kidneys, reduce blood pressure, and decrease intraglomerular pressure. Consequently, proteinuria can be reduced, making these drugs vital for kidney function protection.
Sodium-glucose cotransporter-2 inhibitors (SGLT2is), a newer class of antihyperglycemic agents, reduce glucose reabsorption in the proximal tubules, promoting glucose excretion via urine and thereby lowering blood glucose levels. Studies have shown that SGLT2is can reduce proteinuria in patients with diabetic and non-diabetic kidney diseases and slow the progression of kidney function decline.
In addition to these interventions, managing blood glucose, uric acid, and lipid levels is part of a comprehensive kidney disease treatment strategy.
Prevention and Management of Complications
As kidney diseases progress, various complications may emerge, including hypertension, cardiovascular and cerebrovascular diseases, renal anemia, and bone-mineral metabolism abnormalities. Cardiovascular and cerebrovascular diseases are particularly significant causes of mortality in patients with CKD, who face dual risks of uremia and cardiovascular disease from the onset. These complications not only affect quality of life and life expectancy but can also exacerbate kidney disease progression, creating a vicious cycle that severely impacts prognosis. Early prevention and management of CKD complications are therefore critically important.
Renal Replacement Therapy
Despite active treatment, some CKD patients inevitably progress to end-stage renal disease (ESRD). During severe AKI or at the ESRD stage, maintaining the stability of the internal environment requires renal replacement therapy. Renal replacement therapies include hemodialysis, peritoneal dialysis, and kidney transplantation.
Hemodialysis utilizes an artificial semipermeable membrane to act as the dialysis membrane. Through counter-current flow of blood and dialysis fluid, metabolic waste products are removed from the bloodstream by mechanisms such as diffusion, convection, and adsorption. Hemodialysis also replenishes essential substances like calcium and bicarbonate while removing excess fluid, partially replacing kidney functions.
Peritoneal Dialysis operates on principles similar to hemodialysis but employs the patient's peritoneum as the dialysis membrane instead of an artificial semipermeable membrane.
Kidney Transplantation is regarded as the optimal form of renal replacement therapy, as it not only restores excretory functions but can also reestablish endocrine functions of the kidney. Post-transplantation, patients require long-term use of glucocorticoids and immunosuppressants to prevent and manage rejection.